
A significant paradigm shift is underway in the regulatory environment for impurities in pharmaceutical products. Recent incidents involving genotoxic impurities (GTIs) are driving tighter restrictions and guidelines, as well as more stringent process standards.
As we’ve discussed in previous posts, the discovery of contamination in generic valsartan – despite the scrupulous care of the manufacturer – was a frightening wake-up call that highlighted significant risks to the industry. The impurity had gone undetected for years, demonstrating the need to not only assure the quality of pharmaceutical ingredients, but also the safety of chemical synthesis processes.
This was just one of several recent incidents involving GTIs. Similar cases included the discovery of ethyl mesylate contamination at a Roche plant in Switzerland and EE impurities in the manufacture of Terbinafine. The incidents and others underscored the need for the industry to reevaluate long-standing best practices and safety procedures.
Understanding Drug Impurities
The ICH broadly classifies impurities in one of three groups:
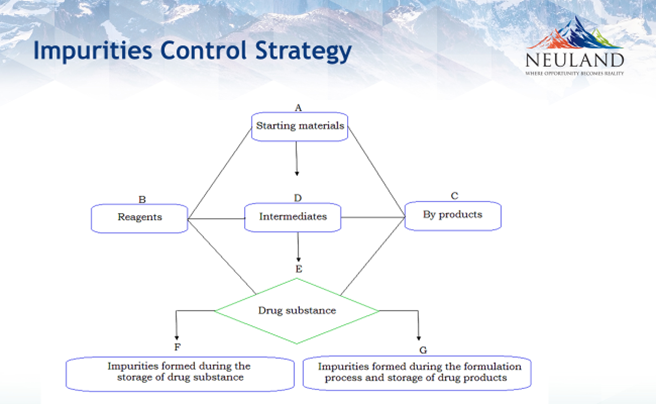
- Organic impurities: starting materials, process-related, intermediates, degradation products and formulation-related impurities
- Inorganic impurities: salts, catalysts, ligands, and heavy metals or other residual metals like Pd, Pt, Ni, Cu, Fe.
- Residual solvents: organic and inorganic liquids used during production.
According to the International Council for Harmonization (ICH), “(G)enotoxic impurities can be broadly defined as impurities that have been demonstrated to cause deleterious changes in the genetic material regardless of the mechanism.” The negative impacts of GTIs may include:
- Mutagenesis: the formation of mutations
- Carcinogenesis: the formation of cancers
- Teratogenesis: damage to the DNA.
Regulators are Cracking Down
In the past, it was sufficient simply to characterize the final product and any associated impurities. In the wake of these incidents, however, regulatory agencies have made significant moves to reduce toxicity risks. Here are just a few of the changes we’ve seen in recent years:
- The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline M7 requires all impurities that have been identified to be considered for their mutagenic potential.
- Effective January 2018, Chapter <231> of the United States Pharmacopeia’s (USP) National Formulary (NF) was replaced by Chapters <232> and <233>. These changes eliminated the traditional “Heavy Metals Test,” which had been an industry standard for more than a century. The new regulations made significant changes to toxicity limits and to the standards for analytical procedures. Notably, toxic substances like lead, mercury, arsenic, and cadmium; as well as metals like platinum, palladium, ruthenium, rhodium, and rubidium; must now be tested and controlled for — even if they are not used in any process.
- In addition, exhaustive GTI assessments and control strategies must now be demonstrated for all products and processes.
 Analytical Challenges
Analytical Challenges
These evolving standards have required API manufacturers to adopt more expensive and cumbersome development and validation strategies.
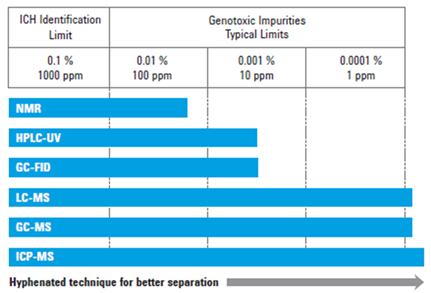
Why? Because unlike typical contaminants, which can be present in 1000, 100, or 10 parts per million (ppm), new toxicity limitations mandate the detection of impurities as low as 1 ppm.
Requiring this level of accuracy knocks a lot of standard lab equipment out of the process. Depending on the nature and quantity of the impurity under investigation, one or more advanced, specialized, and costly techniques may be required, from liquid chromatography–mass spectrometry (LC–MS) to gas chromatography–mass spectrometry (GC–MS) or inductively coupled plasma mass spectrometry (ICP–MS).
Manufacturing Process Challenges
Evolving standards also present new hurdles and concerns at every stage of the manufacturing process. These include:
- Identification of potential impurities throughout the entire route of synthesis, possible byproducts, degradations, etc.
- Redesigning the route of synthesis to eliminate possible GTIs, or to reduce their levels within the scope of the regulatory landscape to acceptable levels of safety and cost.
- Synthesis of all potential impurities and GTIs, their characterization, spiking, and purge studies.
- Development and validation of suitable analytical methods.
- (Q)SAR (quantitative structure–activity relationship) models, in vitro — and sometimes in vivo — evaluation if required to avoid ambiguity.
- Deployment of additional resources, time, cost and effort.
- Possible delays in regulatory approval.
Early Successes with New Solutions
API manufacturers and their partners are working hard to meet these new challenges. In one recent case, a Neuland client’s final API process involved the neutralization of salt with a sodium hydroxide solution. In a related substances test, high performance liquid chromatography (HPLC) detected an unknown impurity at a level of 0.14%.
 To identify the impurity, we used LC–MS. The mass didn’t match any possible structure we could think of that was present in the starting materials, reagents, intermediates or their possible successive structures.
To identify the impurity, we used LC–MS. The mass didn’t match any possible structure we could think of that was present in the starting materials, reagents, intermediates or their possible successive structures.
Subsequently, this impurity was isolated with preparative HPLC. Our investigation of the structure deduced from the isolated material found that it was formed with dichloromethane and acetone in the presence of a strong base. The isolated impurity was spiked with original material to re-confirm it by HPLC, then characterized by nuclear magnetic resonance (NMR) and mass spectrometry. Our hypothesis was further confirmed by lab experimentation.
In another recent case, an unknown impurity — which hadn’t been noticed during the entire development cycle — was detected during process validation. LC–MS analysis revealed an ester hydrolysed product.
Further investigation found that an accidental ammonia leak had occurred during unloading in an adjacent block. The impurity was duplicated in the lab by simulating similar conditions and confirmed by generating structural data and a spiking study.
The Way Forward
Ensuring safe and compliant products and processes will require the industry to take a number of new steps and precautions. We recommend the following practices to minimize your risk:
- Avoid genotoxic reagents or their possible generation by design.
- Allocate the best possible scientists and provide adequate resources for the detection of GTIs. Create a stand-alone cell for this activity.
- Identify all potential structural alerts from the review of systems (ROS) and design a control strategy. Be aware that even this may not ensure “query free” approvals.
- If possible, implement the highest possible levels of impurity control at an intermediate stage, rather that at the API/NCE stage.
- Generate in vitro/in vivo safety data wherever it is difficult to control GTIs or where limits are on the borderline.

Genotoxic Impurities – Raising the Stakes for APIs
The need for greater attention to GTIs has raised the stakes for the entire API community. We’ve already seen multiple cases where impurities have required products to be withdrawn from the market — sometimes in lots or batches, and sometimes forever.
Even when GTIs can be eliminated by adapting a process, manufacturers may need to make considerable unplanned investments and endure time-consuming requalification before a product can be returned to market. Moreover, failure to detect, investigate, and provide corrective and preventive actions for GTIs also increases the risk of critical regulatory attention, such as the FDA’s 483 observations.
As a result, we’re already seeing major investments and manpower deployments in this area, and we anticipate many more. Tightening regulations mean big business for instrumentation companies, especially the makers of LC–MS and ICP–MS equipment. They’re also creating business opportunities for organizations capable of creating new standards and processes for detecting impurities in manufacturing and production.










