Liquid Phase Peptide Synthesis: Guide to Methods, Use Cases, & Strategic Advantages

Liquid phase peptide synthesis (LPPS), also known as solution-phase peptide synthesis, is a classical method for chemically assembling peptides in solution, one amino acid at a time.
In LPPS, the growing peptide stays dissolved in liquid, not attached to resin. Each amino acid addition cycle includes protecting reactive groups, coupling the next amino acid, and purifying the peptide before the next step.
This guide explains what LPPS is, how it differs from solid-phase peptide synthesis (SPPS), and discusses its methodology, advantages, limitations, and uses. It shows how LPPS fits into peptide manufacturing and drug development.
LPPS vs. SPPS – Understanding the Difference
LPPS builds the peptide chain entirely in solution. Each step requires manual extraction and purification before proceeding, making it more labor-intensive. However, it’s still valuable when:
- High purity is essential (purification at every step catches errors early)
- Fragment condensation is needed (combining pre-made peptide segments)
- The peptide is very long or synthesis is complex
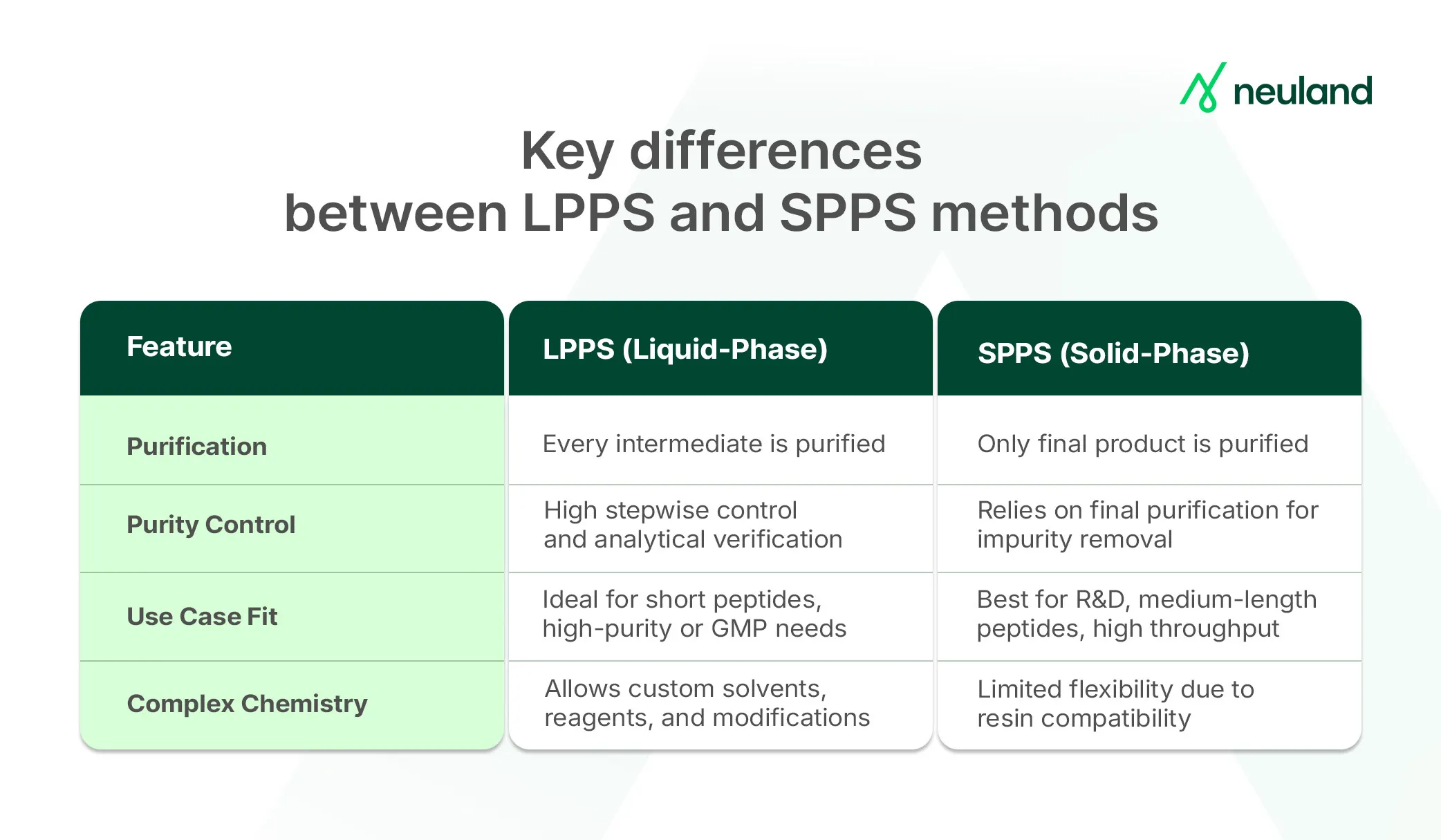
By contrast, SPPS, pioneered by R. Bruce Merrifield in the 1960s, anchors the first amino acid to a solid resin. This allows easy removal of excess reagents after each step via simple washing, eliminating the need to isolate intermediates. SPPS:
- Enables automation and simplifies purification
- Works well for peptides with 50+ amino acids
- Is now the dominant method for R&D due to speed and operational ease
In short, SPPS trades some control for speed and scalability, while LPPS offers meticulous oversight and purity.
Modern peptide manufacturers, including Neuland Labs, often combine methods, using solution-phase techniques for short peptides, SPPS for longer chains, and hybrid approaches for very long or complex sequences. This adaptability helps meet varied project needs efficiently.
How Liquid-Phase Peptide Synthesis Works
In LPPS, peptides are assembled through repeated solution-based chemical reactions following a defined amino acid sequence. Each cycle typically includes:
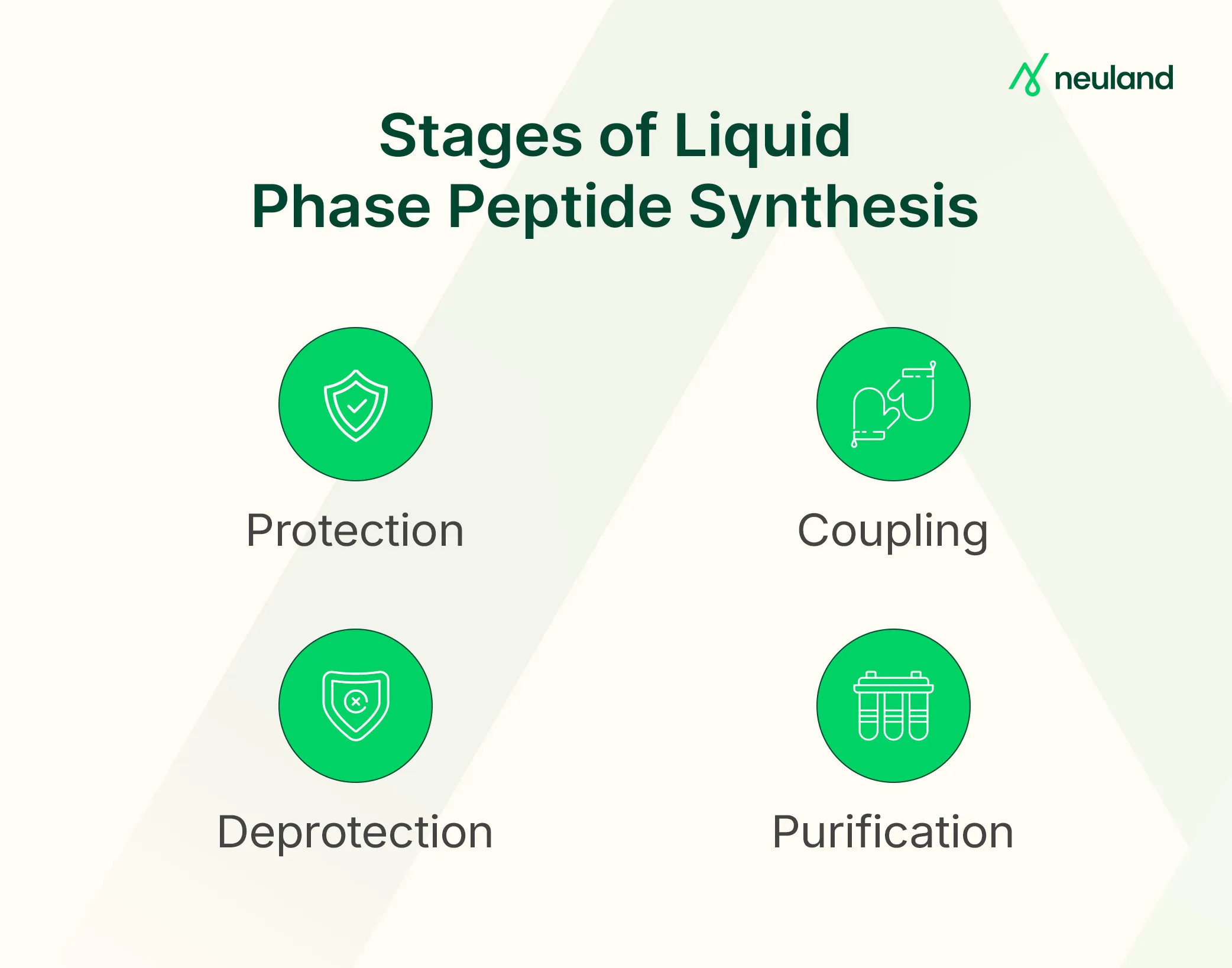
- Protection: Reactive groups on amino acids are temporarily blocked (e.g., Boc or Cbz for the N-terminus) to avoid side reactions. Side chains also carry permanent protecting groups during synthesis.
- Activation & Coupling: The protected amino acid is activated (e.g., with DCC or DIC) and coupled to the peptide chain elongation. This process forms peptide bonds in a C-to-N direction, opposite to natural biosynthesis.
- Deprotection: The temporary N-protecting group on the new residue is removed (e.g., Boc with acid, Fmoc with base), exposing the amine group for the next coupling. The C-terminal remains protected throughout.
- Purification: Unlike SPPS, LPPS requires purification at each step—via extraction, precipitation, or chromatography—to eliminate by-products and confirm intermediate identity (e.g., HPLC or MS).
These steps repeat until the full sequence is built. After final deprotection, the crude peptide undergoes purification (often via HPLC) to reach the required purity.
LPPS can be:
- Linear: Adding one amino acid at a time
- Convergent: Synthesizing short fragments first, then ligating them—ideal for long or complex peptides as it reduces the number of total steps while maintaining control and purity.
Advantages of Liquid-Phase Peptide Synthesis
Despite being more labor-intensive, LPPS offers unique benefits:
- High Purity & Stepwise Control: Since intermediates are purified after every step, side products are caught early. This ensures higher crude purity than SPPS and maintains sequence fidelity—especially useful in regulated environments.
- Better for Difficult or Long Sequences: Through fragment coupling, LPPS handles complex or lengthy peptides by synthesizing and purifying smaller blocks, then joining them. This makes it feasible to produce sequences that challenge standard SPPS.
- Scalable for Simple Peptides: LPPS can be more cost-effective at commercial scale, especially for short peptides. Without resin costs and solvation issues, it supports high-volume production of generics or cosmetic peptides efficiently.
- Greater Chemistry Flexibility: LPPS allows fine-tuned control of solvents, reagents, and conditions—ideal for incorporating unusual amino acids, disulfide bonds, or modifications like PEGylation or cyclization.
- High Yields for Short Peptides: Stepwise purification and precise stoichiometry result in excellent yields for small peptides, making LPPS ideal for tripeptides or other short chains that don’t require solid supports.
Use Cases and Applications of LPPS
Here are some typical use cases and scenarios where LPPS or related solution-phase techniques play a key role:
Bulk Production of Simple Peptides
For small peptides like dipeptides or tripeptides used in cosmetics, nutraceuticals, or intermediates, LPPS enables economical, large-scale production. Its compatibility with large volumes and crystallization makes it ideal for mass manufacturing.
Segment Coupling for Large Peptides
LPPS plays a critical role in hybrid strategies. Segments (10–20 amino acids) are built via SPPS and later joined in solution using chemical ligation, essential for long peptides (>40 amino acids) or those with complex structures like multiple disulfide loops.
GMP-Grade or High-Purity Peptides
When ultra-high purity is required, especially for clinical or regulatory-grade material, LPPS offers tighter control. Purifying intermediates helps avoid side products, making it a preferred method for final-stage GMP manufacturing.
Complex Modifications
LPPS allows chemists to pause synthesis, modify the peptide in solution (e.g., add a lipid chain, fluorophore, or non-standard amino acid), purify the intermediate, and continue. This flexibility supports custom synthesis needs beyond SPPS’s limitations.
Conjugation and Ligation Applications
LPPS enables solution-phase couplings like peptide-drug or peptide-polymer conjugations. Methods such as native chemical ligation also occur in solution, making LPPS vital for assembling therapeutic conjugates and engineered peptides.
Choosing the Right Peptide Synthesis Method and Partner
Liquid phase peptide synthesis remains an important technique in the peptide chemist’s arsenal. It offers unparalleled control and can achieve exceptional purity, which is crucial for certain applications in biotech and pharma. However, it also requires considerable effort and expertise.
If you’re evaluating custom peptide synthesis services or planning to scale up a peptide drug candidate, it’s wise to work with a partner experienced in all techniques. Neuland Labs has 4 decades of peptide manufacturing know-how and capabilities spanning solution-phase, solid-phase, and fragment condensation methods.
By offering end-to-end custom peptide synthesis services from milligram-scale research peptides to multi-kilogram GMP production, Neuland Labs can apply the approach that best fits your project’s requirements.
FAQs
|
|
|
|