
From IND to Commercial: How Neuland Ensures Phase-Appropriate Development

What Is API Process Development? Key Steps and Best Practices

CMC Development for Peptide APIs: Key Considerations

Speed and Flexibility: The Defining Metrics for Finding a CDMO You Can Trust

Analytical Method Validation: Key Parameters & Common Challenges

A CDMO’s Guide to Process Development & Scale-Up

ESG in Pharma: How CDMOs Drive Sustainable Supply Chains

How a Boutique CDMO Differs from a Full-Service CDMO

Understanding the Drug Substance Manufacturing Process: Key Steps & CDMO Role

What Is Drug Substance Manufacturing and Why Is It Critical in Pharma?

What Trends Are Transforming the CDMO Industry in 2025 and Beyond

Why Early Manufacturing Process Optimization is the Safest Path to Efficient Scale-Up

What Is a Boutique CDMO? Key Characteristics & Role in Pharma
Custom Peptide Synthesis: Choosing the Right Method for Your Sequence

Custom Peptide Synthesis Partner: Key Factors for Selecting the Best Fit

What Is Clinical Manufacturing? A Guide to Processes, Regulations, and Outsourcing

CDMO vs CMO vs CRO: Choosing the Right Outsourcing Partner in Drug Development

API Micronization in the Early vs. Late Stages of Drug Development: Goals and Challenges

From Milligrams to Kilograms: Scaling Up Synthetic Peptides

CDMO Market Outlook 2025–2030: Trends Shaping the Future of Pharma Outsourcing
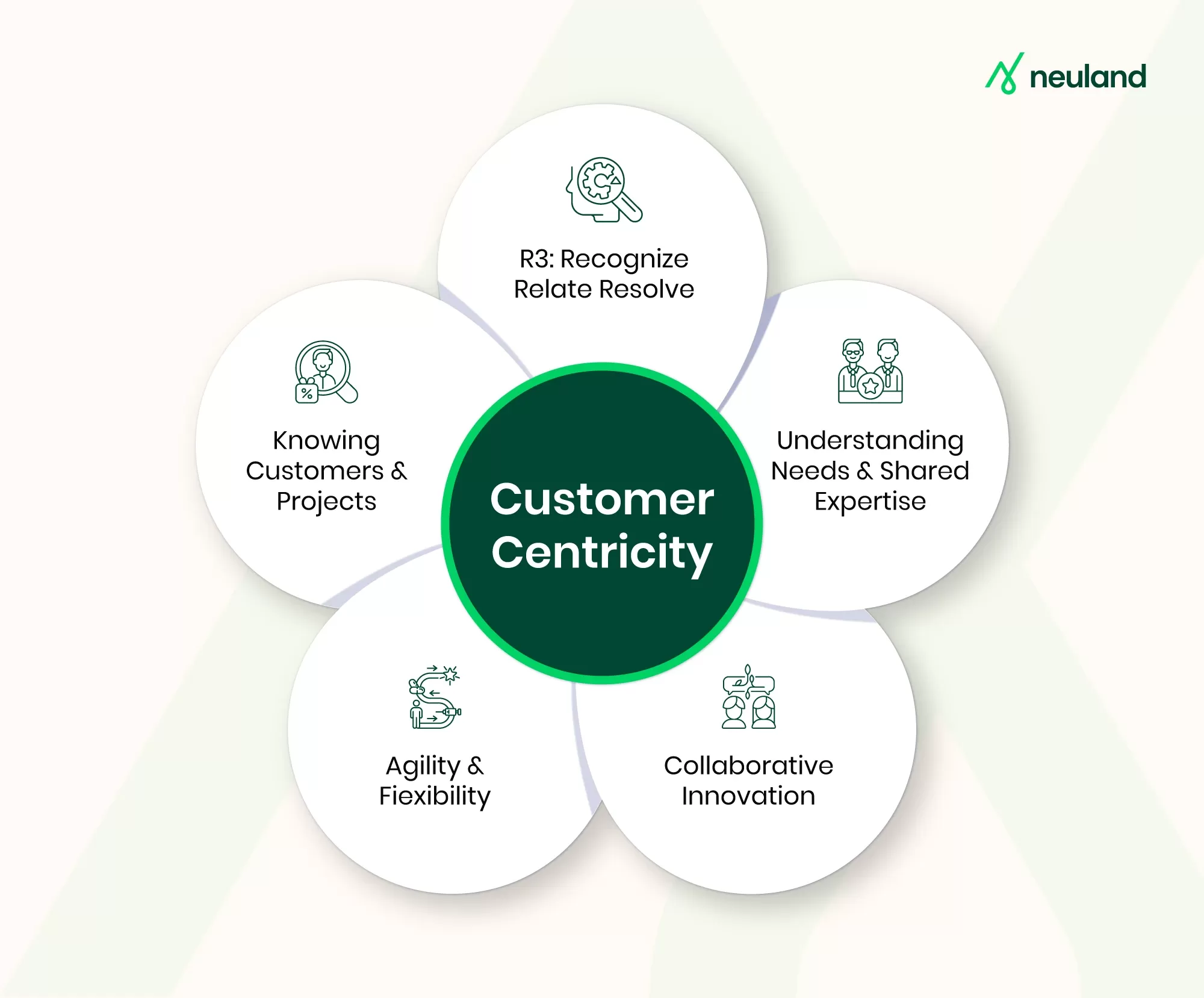
Why a Customer-First Mindset Is the Key Driver of Innovation in CDMOs

Peptide Manufacturing at Commercial Scale: What to Expect from a Trusted CDMO Partner

Liquid Phase Peptide Synthesis: Guide to Methods, Use Cases, & Strategic Advantages

Peptide Conjugation - Common Conjugation Strategies

CDMO vs. CMO: Understanding the Outsourcing Models That Drive Drug Development

Peptide vs. Protein: Breaking Down 5 Essential Differences for Drug Makers

A Complete Guide to Commercial Manufacturing for Pharma Innovators

Your Guide to CDMO Services: What Leading Pharma Teams Rely On

Building a More Sustainable Supply Chain in Pharma

Why the CDMO Industry Matters More Than Ever in Drug Development

Small Molecule Drug Development: Process, Strengths, and CDMO Role

What Is Solid Phase Peptide Synthesis? Process, Uses & Benefits

Business Continuity Management—the Next Frontier in API Supply Chain Security

How CDMOs Help Biotech Advance and Navigate Drug Development and Manufacturing Complexities

The Ultimate Guide to Selecting a CDMO Partner

Understanding cGMP Compliance for Pharmaceutical Manufacturing Excellence

Why Pharma Giants Are Outsourcing Drug Manufacturing to CDMOs

All You Need to Know About Outsourcing Drug Manufacturing & Development to CDMO

5 Reasons Why More Pharma Companies Are Strengthening CDMO Relationships

Quality Risk Management in Pharmaceutical Industry

Neuland Announces New Vision and Values Statements

The Essential Role of Intellectual Property Expertise in a Pharmaceutical API Company

Achieving Drug Development Success: Inside Neuland’s Three-Pillar Project Management Strategy

From the Neuland Reading Room: Peptide Trends in 2024

Zero Liquid Discharge: A Pharma Industry Commitment to Environmental Sustainability

The Pharma Supply Chain: Reshaping Itself for The New Normal

Redefining Excellence: Neuland Unveils a Refreshed Brand Identity

From the Neuland Reading Room: A Check-in on 2024 Trends in the CDMO Industry

How to Ensure Effective Technology Transfer to CDMOs

Why Agility Matters When Choosing an API Contract Manufacturing Partner

API Particle Size Part 2: New & Emerging Technologies Impacting Particle Size

API Particle Size Part I: Overcoming the Challenges to Ensure Quality Pharmaceutical Products

Scaling-up Active Pharmaceutical Ingredient (API) Chemistry for Phase 3 Clinical Trials and Bulk Manufacturing for a Potentially Novel Treatment for Schizophrenia

R&D Expert Talks Pharma API Scale Optimization

The Crucial Role of Crystallization in Drug Substances Development

What Pharmacological Advantages Can Deuterated APIs Deliver?

Integrating Sustainability into Pharma Manufacturing

8 Ways Effective Supply Chain Management Can Minimize Pharmaceutical Supply Chain Challenges

Advancing a Cholesterol Lowering Drug at the Height of the Pandemic

ESG in Pharma: Building Sustainable Businesses

How Neuland’s Process Engineering Lab Supports Robust Design and Minimizes Risk

Putting Customers First: Customer Centricity in Pharma API Manufacturing

3 Key Elements of a Successful Hydrogenation Scale-Up

Why is Pharmaceutical Green Chemistry Use on the Rise?

Regulatory Starting Materials: How to Source, Analyze and Validate Suppliers (Part I)

Exploring Neuland’s Therapeutic Segments: Focus on CNS

Green Chemistry by Design (GCbD): The Earlier, The Better for Pharmaceutical APIs

Deuterated Drug Molecules: Perfecting the Gamechanger

What Does it Take to Become a Specialist in Late-Phase CMS?

API Production: Building a Process Safety Culture

Looking for an API Supplier? 5 Things to Consider (Updated)

Nitrosamine Impurities and How Companies are Addressing the Crisis

Successful Pharmaceutical Project Management

Complex Synthesis in Action: Achieving a Commercial-Scale 16 AA Peptide

A Guide to Sourcing Pharmaceutical Peptide APIs

Case Studies from Neuland’s Process Engineering Lab

Overcoming Challenges in Complex Peptide Purification

Welcome, Unit 3!

QbD and Evaluating ‘What-If’ Drug Manufacturing Scenarios

NCEs Versus Generics – Adjusting Project Tactics & Objectives to Maximize Success

Synthetic Route Scouting: Factors to Improve API Manufacturing

How to Select a Peptide Synthesis Technique

Leveraging QbD for API Scale Up

5 Common Challenges Scaling Up an API

How Process Engineering Techniques Result in Cost-Effective Products

API Manufacture of Deuterated Molecules

Advantages of a Pure-Play API Contract Manufacturer

APIs & Continuous Flow Manufacturing

Challenges with API Micronization Techniques for Use in Injectables and Medical Devices

Advances in the Synthesis of Peptide Therapeutics: Clear Advantages…and Some Barriers

Genotoxic Impurities – Increasing Vigilance, But Still Some Uncertainty

6 Properties of API Synthesis that Affect Yield, Delivery Date & Purity

Contract Drug Manufacturing R&D: from Route Scouting to Kilo Labs

The Benefits of Synthesis Scouting

Analytical Capabilities and Drug Quality – Key to Successful Pharma Outsourcing

The New Look of Neuland

Peptide Synthesis: Solution Phase, Solid Phase or Hybrid?

Neuland Labs Receives 2013 CMO Leadership Award for Regulatory Excellence
