Custom Peptide Synthesis Partner: Key Factors for Selecting the Best Fit
A promising peptide API is only as strong as the partner you trust to synthesize it. The wrong choice can mean delays, inconsistent purity, or regulatory setbacks.
However, selecting the right custom peptide synthesis partner is a strategic decision that requires time and understanding of factors. Whether you need research-grade peptides or GMP-grade peptide APIs, the provider must balance technical capabilities, quality controls, and reliable service.
In practice, your choice hinges on project specifics and on the partner’s ability to meet both R&D and clinical‐manufacturing requirements. Therefore, let’s discuss the key factors to guide your selection.
Define Your Peptide Service Requirements
First, clearly define your peptide needs. Consider peptide length, sequence complexity, and any unusual or non-natural amino acids.
Determine required purity and quantity. Note any special modifications such as PEGylation or conjugations. Having precise specs helps you communicate with vendors.
It also clarifies which type of service you need: for early R&D, speed and flexibility usually top the list, whereas for GMP peptide API production, the emphasis shifts to validated processes, rigorous documentation, and regulatory compliance.
Key Factors to Assess When Choosing a Custom Peptide Synthesis Partner
The following are the key factors you should assess in order to choose the right custom peptide services:
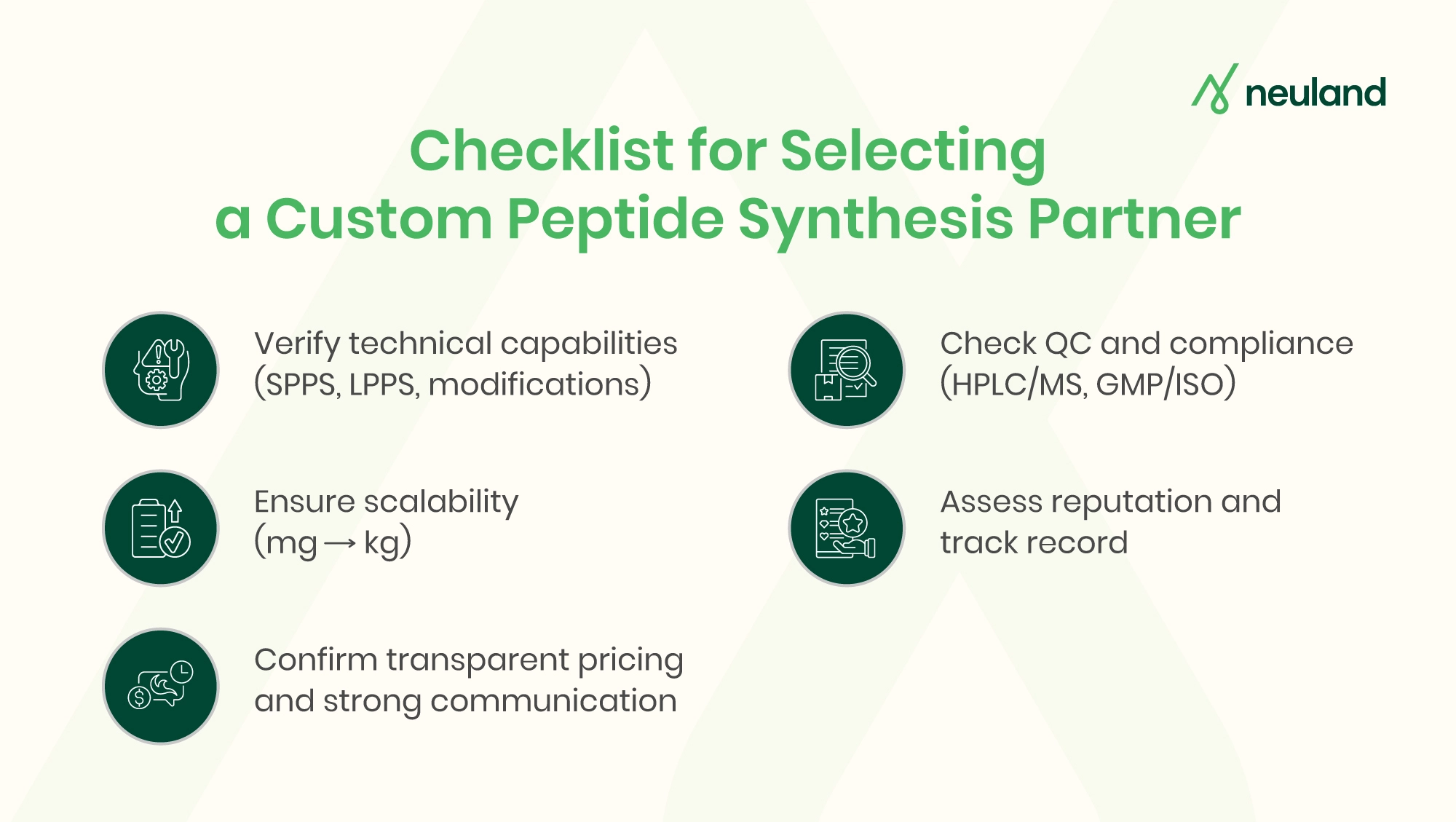
Technical Capabilities and Customization Options
Evaluate the provider’s synthesis technology and expertise. A strong partner should offer large-scale peptide synthesis and support complex chemistries.
Look for companies experienced with long peptides, cyclic or stapled peptides, and incorporation of D-amino acids or other unusual residues.
They should also handle post-synthesis modifications—phosphorylation, acetylation, PEGylation, lipidation, fluorescent tags, carrier conjugations, etc.
Quality Control and Compliance Standards
Quality is non-negotiable. Your partner must provide rigorous analytical support to verify identity and purity. Standard practices include HPLC chromatography and mass spectrometry for every batch.
Ensure they supply full certificates of analysis (COAs) or detailed reports showing purity and characterization data. Reputable CDMOs typically achieve peptide purities in excess of 98% for critical applications. For in vivo or clinical-stage peptides, additional tests may be necessary. Moreover, verify that the company maintains formal quality systems.
Look for ISO certifications (9001, 13485) and, if producing APIs, cGMP-compliant facilities. A partner with certified, audited plants and validated processes will ensure each batch is consistent and traceable.
Scalability and Manufacturing Capacity
Many R&D projects begin with milligram or gram quantities, but successful pipelines often require escalation to hundreds of grams or kilograms for clinical trials and commercialization. Leading providers emphasize scalable operations.
Ask about their past projects – have they produced kilos of peptide APIs? Do they run larger-scale reactors or employ fragmented synthesis strategies for longer sequences?
Ensure the custom peptide synthesis partner’s capacity and equipment match your anticipated output, from pilot batches through commercial volumes.
Experience, Support, and Collaboration
Experience in peptide chemistry is invaluable. Years in the field usually mean a higher success rate and quicker troubleshooting. Inquire about the team’s expertise: do they have in-house peptide chemists and process development scientists?
Good partners act as an extension of your team. They should offer scientific consultation—helping with sequence design, solubility/stability issues, and synthesis strategy.
It’s also wise to assess “culture fit”: the partner’s working style and mindset should align with yours. As one biotech client observed when choosing a CDMO, transparency, expertise, and a collaborative attitude were decisive factors.

Reputation and Track Record
A provider’s reputation is a window into reliability. Look for companies with proven track records in peptide synthesis – especially those with existing pharma or biotech clients.
Ask for references or case studies relevant to your project. Check independent reviews or publications if available. Peer recommendations are very informative; colleagues in your field may have direct experience with certain peptide services.
Also verify the provider’s consistency: do they guarantee batch-to-batch uniformity and robust quality protocols? If possible, choose partners who already work in or near your therapeutic area, since familiarity with specific peptide types or regulatory requirements can be an advantage
Cost, Transparency, and Value
Price is important but should not compromise quality. Seek detailed, transparent quotes that break down all costs.
Beware of hidden charges or too-good-to-be-true pricing that might signal cut corners. Instead of picking the cheapest option, consider value for money: paying a bit more to ensure higher purity, better documentation, and reliable timelines is often worth it.
Many providers offer discounts for bulk orders or long-term contracts, so discuss volume pricing if you expect multiple batches. Remember that a failure or delay can cost far more than a premium service, so weigh price against assurances of quality and service level.
Communication and Project Management
Effective communication is essential. Even a technically capable partner can create headaches if they’re unresponsive or opaque. During evaluation, note how quickly they answer questions and how clearly they explain their processes.
Throughout the project, you’ll want regular status updates and prompt responses to any issues. Ask about their project management practices:
- Will you have a dedicated scientist or project manager?
- How do they handle problem resolution if a synthesis fails or results are off-spec?
Reputable custom peptide synthesis partners often have protocols to address such scenarios. Also, check post-delivery support, as good after-sales support can save time and ensure your project stays on track.
A Custom Peptide Synthesis Partner with Integrated Services
A top-tier custom peptide synthesis partner will meet your technical needs and quality standards while acting as a true collaborator.
Neuland Labs, as a global CDMO with four decades of experience in peptide chemistry, offers integrated custom peptide synthesis services, from early-stage development to GMP-grade peptide API manufacturing.
If you plan on using a specialized CDMO for your peptide synthesis needs, Neuland delivers a strategic partnership for companies advancing peptide-based therapeutics with proven regulatory compliance and advanced facilities. Contact us today.
FAQs
|
|
|
|