CDMO vs CMO vs CRO: Choosing the Right Outsourcing Partner in Drug Development
Drug development is a complex, multi-stage journey from initial discovery through clinical trials to full-scale manufacturing and market launch.
At each stage, pharmaceutical companies often turn to external pharmaceutical outsourcing companies for support, tapping into pharmaceutical contract services to fill capability gaps. These partners typically fall into three categories:
- Contract Development and Manufacturing Organizations (CDMOs)
- Contract Manufacturing Organizations (CMOs)
- Contract Research Organizations (CROs)
The global pharmaceutical outsourcing market is booming — but with so many players competing as CDMO vs CMO vs CRO, knowing which one fits your needs can mean the difference between delay and speed-to-market.
Below, we break down each type of partner, compare their roles, and outline how to choose the best fit for your drug development needs.

What is a CRO?
A CRO is a service provider that specializes in the research and clinical trial phases of drug development.
CROs manage early-stage development tasks such as designing and running clinical trials, performing preclinical studies, handling data management, and navigating regulatory submissions.
In practice, a CRO might take charge of clinical trial services – planning the study protocol, recruiting patients, monitoring trials, analyzing data, and ensuring compliance with regulations. CROs do not manufacture drugs; rather, they provide the scientific and logistical support needed to demonstrate a drug’s safety and efficacy.
What is a CMO?
A CMO focuses on the drug manufacturing stage. CMOs provide the infrastructure, equipment, and trained personnel to produce drug substances and finished dosage forms at scale.
In the drug development timeline, a CMO usually comes into play once a drug candidate is developed and ready for large-scale production or late-stage clinical trial material manufacturing.
Services offered by CMOs typically include process scale-up, commercial batch production, packaging, and quality control testing. They adhere strictly to Good Manufacturing Practice (GMP) standards to ensure the final product meets all quality and regulatory requirements.
What is a CDMO?
A CDMO offers an integrated set of services that span both contract development and manufacturing.
In essence, a CDMO can support a drug project from early development through process optimization, clinical trial material supply, and ultimately commercial manufacturing.
This approach means pharmaceutical companies can rely on a single partner for end-to-end development and production, rather than handing off the project between separate R&D and manufacturing vendors.
Integrated CDMO services commonly include process development, analytical method development, API development and manufacturing, scale-up of production processes, supply chain management, and even regulatory support.
Additionally, in the CDMO vs CRO discussion, some CDMOs have expanded their offerings to cover certain research activities as well by acquiring CRO capabilities.
CDMO vs CMO vs CRO: Core Differences
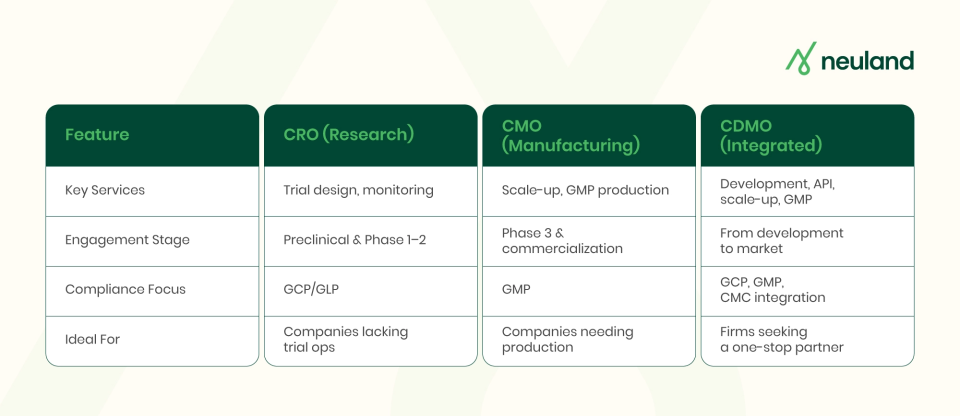
While CDMO vs CMO vs CRO are all a part of the pharmaceutical outsourcing ecosystem, they serve different primary functions and stages. The following points summarize the key differences and overlaps among the three:
-
Primary Focus
CROs focus on research and trials, designing studies, managing operations, and handling data/regulatory tasks. CMOs specialize in manufacturing, producing drugs at scale with quality control. CDMOs combine development and manufacturing, covering the entire drug lifecycle from formulation to large-scale production, offering a hybrid of CMO and development capabilities.
-
Scope of Services
A CRO handles protocol design, trial monitoring, data analysis, and regulatory support. CMOs focus on process scale-up, production, packaging, and GMP testing. CDMO solutions range from development to commercial production, combining roles of CRO and CMO.
-
When They Engage
CROs are engaged early in preclinical and Phase 1-2 trials for data and filings, while CMOs typically come later for manufacturing during Phase 3 or commercialization. CDMOs can be involved throughout, developing processes for early phases and scaling up for launch, ensuring continuity within one organization.
-
Regulatory Compliance
All three partner types are compliance experts in different areas. CROs ensure GCP and GLP standards during trials and lab studies. CMOs and CDMOs focus on GMP compliance for production. CDMOs also have expertise in Chemistry, Manufacturing, and Controls (CMC), bridging development data to manufacturing.
It’s worth noting that the lines between these categories can blur. Some organizations label themselves interchangeably as a CDMO vs CMO, and many large service providers offer multiple capabilities.
However, the distinctions above remain useful for understanding what your project needs: research expertise, manufacturing capacity, or an integrated solution.
Choosing the Right Partner Between CDMO vs CMO vs CRO
Selecting a CDMO vs CMO vs CRO comes down to your company’s specific needs, stage of development, and strategic goals. There is no one-size-fits-all answer, and in fact many pharmaceutical firms will work with multiple partners over a drug’s development timeline.
Below are key factors and considerations to guide the decision:
- Stage & scope of needs: Identify which phase of the drug lifecycle you need help with. In many cases, a biotech startup might use a CRO for early studies, then transition to a CMO or CDMO when it’s time for production
- Timeline and speed-to-market: Consider your timelines and where you need to accelerate. If timing is critical, a CDMO partner might prevent delays that occur when transferring a project from one partner to another
- Resources and Infrastructure: Evaluate your in-house capabilities. Choose based on whether you need an operations team or manufacturing facilities or both
- Risk management and expertise: Different partners help mitigate different risks. If avoiding the risk of misaligned hand-offs or gaps in responsibility is a priority, CDMO solutions might be the safest bet as an all-in-one partner
- Strategic flexibility: It’s also possible to adopt a hybrid approach. However, always ensure clear communication and quality agreements are in place when multiple parties are involved
CDMO vs CMO vs CRO: The Final Word
Choosing between a CDMO vs CMO vs CRO is a pivotal decision that can define the trajectory of a drug development program.
The right choice depends on your stage of development, available resources, and timelines. In many cases, success comes from working with different partners at different stages as a molecule progresses from concept to market.
Ultimately, when comparing CDMO vs CMO vs CRO, the goal is to build collaborative relationships with partners who act as an extension of your team. An experienced, quality-focused organization ensures projects move forward efficiently, compliantly, and with minimal risk.
At Neuland Labs, we bring over four decades of experience as a global CDMO, with specialized expertise in small molecules and peptides.
By combining proven compliance, technical depth, and integrated solutions, we help accelerate development and manufacturing programs. To explore how our expertise can support your next project, connect with us today.
FAQs on CDMO vs CMO vs CRO
|
|
|
|