The Ultimate Guide to Selecting a CDMO Partner
From concept to commercialization, your CDMO partner defines the success of your product’s journey.
An ideal partner accelerates timelines, enhances product quality, navigates complex regulations smoothly, and aligns perfectly with your strategic objectives.
However, making the wrong choice can lead to regulatory roadblocks, production delays, and substantial financial losses. That's why it's essential to understand the key factors that set a truly exceptional CDMO apart.
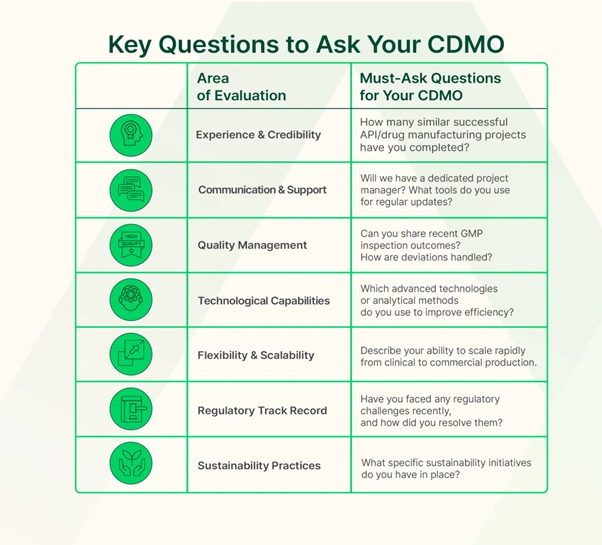
1. Experience and Industry Credibility
When vetting a CDMO, start by assessing their experience and track record in the pharmaceutical industry.
Evaluate a CDMO’s credibility through its proven track record—measured by years of operational excellence, the volume of successful compound deliveries, and longstanding partnerships with leading pharmaceutical companies.
Industry credibility also means the CDMO partner has a strong reputation for reliability and competence. Check if they can provide case studies or references demonstrating past successes, such as meeting difficult process requirements or achieving timely scale-up for other sponsors.
2. Comprehensive Support and Communication
Effective CDMO pharma partnerships involve comprehensive support across all development stages and clear communication at every step.
An ideal CDMO partner should demonstrate seamless end-to-end capabilities—spanning early-stage process development, formulation, clinical trial material production, and scalable commercial manufacturing.
In practice, this means fewer hand-offs, better knowledge transfer through the stages, and a partner who can adapt as your project advances.
Efficient project execution depends on proactive communication and structured project oversight. For instance, Neuland Labs’ comprehensive three-pillar project management strategy ensures full transparency through transparent communication, proactive risk management, and agile adaptation to project dynamics.
3. Quality Assurance
A reputable CDMO must demonstrate an unwavering commitment to quality assurance (QA) in every aspect of development and manufacturing.
- Your CDMO must strictly follow global Good Manufacturing Practices (GMP) standards, which include validated methods, robust documentation, trained personnel, and defined procedures for handling deviations and out-of-specification (OOS) events.
- Look for tangible proof of quality—FDA/EMA audit outcomes, GMP certifications, and internal metrics like batch success and right-first-time rates.
- Opt for a CDMO that integrates quality into its core—leveraging QbD principles, risk-based thinking, and proactive systems to prevent issues and drive consistent performance.
Key differentiators include how the CDMO approaches process validation and equipment qualification. These steps ensure that every scale-up or process change yields consistent results batch after batch.

4. Advanced Tools and Technologies
Pharmaceutical science and engineering are continually evolving, from automation and robotics to new processing techniques, and your CDMO partner should be keeping pace or even leading in innovation.
CDMOs that implement process intensification methods, like continuous manufacturing or advanced purification techniques, often achieve higher yields, better purity, and faster production cycles.
When evaluating this aspect, ask what technical capabilities and specialized equipment a CDMO offers.
- Do they have high-throughput screening tools for formulation?
- For scaling, are they using automated reactors or fermenters?
- What are the proprietary technologies that give them an edge in novel chemistries or biologics production?
5. Flexibility and Scalability
The ideal CDMO partner will work with you to adjust batch sizes, modify protocols, or expedite certain activities as your needs evolve.
Look for indicators of flexibility, like a range of class sizes (from small R&D batches to larger pilot runs), customizable services, and a project team that’s open to iterative development rather than a one-size-fits-all approach.
A CDMO should have the capacity and infrastructure to scale your project from grams in the lab to kilograms or tons on a commercial scale without hiccups. Assess their manufacturing facilities:
- Do they have multiple production lines or large-scale reactors to ramp up output when needed?
- How do they internally handle tech transfer from development to production?
The best partners design processes with scale-up in mind and often have experience taking molecules from clinical trial quantities to full market supply. Neuland Labs has a strong track record in successful API technology transfers, backed by deep expertise in problem-solving across complex transfers.
6. Regulatory Expertise
When evaluating a CDMO-pharma partnership, examine their understanding of the regulatory landscape relevant to your product: Do they keep up with FDA, EMA, and ICH guidelines, and have they successfully supported filings (INDs, NDAs, DMFs) in major markets?
An ideal CDMO, such as Neuland Labs, will have a dedicated regulatory affairs team or experts who can guide CMC (Chemistry, Manufacturing, and Controls) documentation, assist in writing regulatory submissions, and ensure all processes meet current standards.
Ask how the CDMO stays current with changing regulations or guidance, such as new impurity guidelines or evolving GMP standards. Do they conduct regular internal training and audits to preempt compliance issues?
7. Commitment to Sustainability
Choosing a CDMO partner with strong sustainability initiatives means fewer risks of future compliance issues (as regulations tighten on environmental emissions, for instance) and supports your brand’s image as a forward-thinking organization.
When evaluating CDMOs, look into their environmental practices: Do they implement green manufacturing principles such as waste reduction, recycling, or solvent recovery?
- Check if the CDMO is adopting measures to minimize environmental impact, such as energy-efficient processes, greener reagents, and advanced waste treatment.
- Look for corporate sustainability commitments, such as setting targets for emissions reduction or water conservation, as these indicate that the partner takes sustainability seriously across its operations.
- Consider social aspects such as worker safety programs, community engagement, or ethical supply chain practices (sourcing raw materials responsibly).
- Assess whether the CDMO publishes sustainability reports or has relevant certifications, such as ISO 14001 for environmental management.
A leading CDMO partner such as Neuland Labs has three worldwide compliant facilities and is certified by WHO, US FDA, EMA, PMDA, ISO 14001, ISO 27001, and OHSAS 18001.
Final Thoughts: Choosing Your Ideal CDMO Partner
By carefully evaluating each of the seven critical parameters outlined in this guide, organizations can identify a partner that aligns with both their technical requirements and strategic vision.
Neuland Labs stands out as a CDMO that consistently meets these criteria. With a decades-long legacy in API development and manufacturing, the company brings together robust GMP compliance, proven regulatory track record, and advanced process technologies.
By collaborating with Neuland Labs, pharmaceutical businesses gain more than execution support—they gain a strategic ally equipped to navigate complexities and accelerate development with confidence.
FAQs
|
|
|
|