All You Need to Know About Outsourcing Drug Manufacturing & Development to CDMO
In 2024, AstraZeneca abandoned a £450 million vaccine plant expansion in the UK due to shifting government policies.
It was a glaring example of the industry's unpredictable nature. Even a pharmaceutical giant with deep pockets couldn’t justify the risks of building a massive in-house manufacturing facility.
Developing a new drug is a high-stakes and high-cost endeavour with some major challenges:
- Changing global policies can delay or derail production
- One missing ingredient can bring development to a halt
- Building facilities and maintaining compliance is expensive and unpredictable
Nowadays, more biotech and pharma firms are outsourcing drug development and production to specialized partners such as Contract Development and Manufacturing Organizations (CDMOs).
This guide goes beyond defining what a CDMO is—it will discuss in depth how you can strategically leverage these organizations to reduce risk, streamline operations, and accelerate drug development.
But First, A Quick Look at CDMO vs. CMO vs. CRO
Let’s clear up the confusion: How do CDMOs, CMOs, and CROs differ—and why does it matter for your business?
- CDMO (Contract Development & Manufacturing Organization): A full-service partner handling both drug development and manufacturing, from early-stage formulation to large-scale production.
- CMO (Contract Manufacturing Organization): Focuses only on manufacturing, helping companies scale production without investing in their own facilities.
- CRO (Contract Research Organization): Specializes in research and clinical trials, supporting drug discovery and regulatory approvals.
| Organization Type | Core Focus |
| CDMO (Contract Development & Manufacturing Organization) | Full-service partner for both drug development and manufacturing |
| CMO (Contract Manufacturing Organization) | Manufacturing-only service provider |
| CRO (Contract Research Organization) | Research-focused partnerships for preclinical and clinical studies |
Why Your Biggest Bet Isn’t In-House—It’s Outsourced
With increasing complexities in drug formulations, moving a treatment from lab to market requires precision, expertise, and speed.
CDMOs bring the expertise and infrastructure, from advanced synthesis techniques to commercial-scale production, needed to handle challenges that would otherwise slow you down.
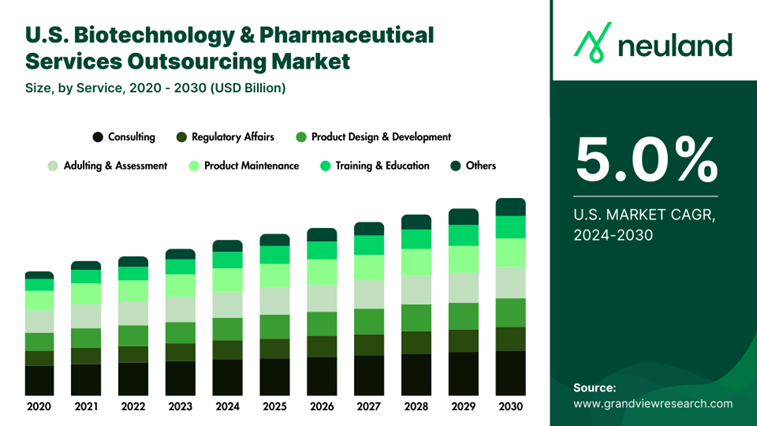
Even big pharma leans on CDMOs to optimize processes, expand capacity, and stay ahead in a hypercompetitive market.
By applying agile working methods, CDMOs can help you achieve up to a 30% reduction in costs while improving quality and accelerating the delivery of products and services, in some cases, by up to 100%.
The best CDMOs are utilizing AI-driven process optimization, cutting-edge bioreactors, and proprietary manufacturing efficiencies that turn years into months and months into weeks.
Why You Need a CDMO Today: Addressing Modern Challenges
The pharmaceutical landscape is changing fast—regulatory uncertainty, AI integration, and expanding global markets are all on the horizon. With the right CDMO, you can ensure your business remains competitive and compliant.
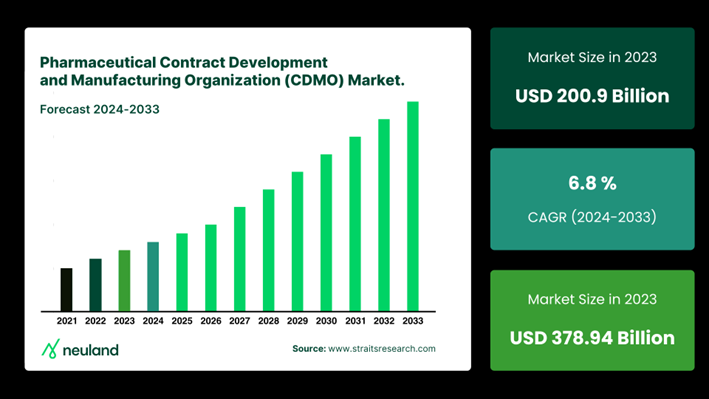
Regulatory Uncertainty: The BIOSECURE Act's Ripple Effect
The BIOSECURE Act of 2023 is the latest example of regulatory uncertainty. Aimed at reducing U.S. reliance on Chinese supply chains, the act calls for a gradual shift away from Chinese manufacturers by 2032.
Companies are already scrambling to diversify their supply chains, even if the act’s provisions don’t come into full effect.
Sarah Karlin-Smith's insightful piece on Citeline’s Pink Sheet, “Inertia for Supply Chain May Stick Even If BIOSECURE Fails,” highlighted that, even if the BIOSECURE Act falters, the industry is in the midst of a deeper transformation.
Businesses are reevaluating their suppliers, questioning long-standing dependencies, and exploring contingency plans. For you, this means staying one step ahead, finding regulatory-compliant partners who can pivot quickly to avoid future bottlenecks.
This is where CDMOs come in handy. With their experience navigating complex regulatory landscapes, CDMOs help mitigate the risks of such uncertainties and keep your production schedule intact.
AI/ML Integration: The Digital Revolution in Drug Manufacturing
Leading CDMOs are integrating continuous manufacturing, advanced automation, and real-time data analytics.
For instance, they are tapping into Continuous Flow Reactor technology, which enhances process precision and minimizes resource consumption - a big win for efficiency and sustainability.
This means your operations will be more connected, transparent, and—importantly—more scalable. With AI-driven insights, CDMOs are already optimizing drug development timelines, reducing errors, and boosting output.
Expanding into New Markets: Seamless Global Expansion with CDMOs
Breaking into new markets means dealing with layers of bureaucracy, regulatory mazes, and logistical headaches that can slow you down. One misstep, and your drug could be stuck in approval purgatory while competitors race ahead.
This is where multinational CDMOs become even more important. They’ve been through the gauntlet and know how to navigate different regulatory bodies without the usual bottlenecks.
More importantly, they make technology transfer seamless, so whether you're producing in North America, Europe, or Asia, you don’t have to reinvent the wheel every time you scale up.
Peptide-Related Opportunities: Tapping into the Rising Demand for Biologics
The global peptide-based drug market is projected to reach a staggering $5 billion by 2030, growing at an 11.3% CAGR from 2024 onward.
With peptides playing an increasingly critical role in oncology, metabolic disorders, and autoimmune diseases, pharmaceutical and biotech companies are intensifying their focus on peptide-based therapies.
Beyond these sectors, peptides are driving breakthroughs in gene therapy, diagnostics, and biomarker research. From cell-penetrating peptides enabling advanced drug delivery to peptide-based biosensors revolutionizing disease detection, their versatility is expanding across multiple fields.
However, scaling peptide production demands cutting-edge synthesis techniques and stringent quality control—areas where specialized CDMOs excel. With capabilities like large-scale cGMP manufacturing and analytical validation at each step, the right CDMO ensures seamless scalability without compromising purity or compliance.
Managing Risk While Scaling Manufacturing: CDMOs as Your Flexible Growth Partners
Shifting production volumes or increasing batches to meet rising demand might send your operation into chaos. Fail to meet production deadlines or compromise on quality, and you're staring at major setbacks and costly delays.
CDMOs can help you stay lean and nimble while accessing full-service support at every stage of development. From clinical trials to commercial production, CDMOs offer flexibility that’s hard to replicate with in-house manufacturing.
The key advantage will be their speed and efficiency. By handling scale-up support, a CDMO ensures that you can meet demand without jeopardizing quality, all while staying within budget.
So, rather than pouring time and resources into building an internal infrastructure, partnering with a CDMO frees you up to focus on what really matters: drug discovery, marketing, and long-term success.
The Unparalleled Benefits of Working With a CDMO

Higher Yield
In drug manufacturing, every extra percentage point in yield isn’t just a number—it’s time, money, and efficiency gained. CDMOs specialize in process optimization, identifying inefficiencies and tweaking methods to maximize output.
For instance, Neuland Labs transformed a 34% yield into 61% for a biopharma client, cutting production cycles and material waste. As a result, a safer and more efficient process was implemented, and a critical milestone was achieved at a fraction of the anticipated cost.
Higher Savings
Owning a manufacturing facility sounds great—until you factor in the real costs. Equipment, staffing, compliance, maintenance—building and running an in-house operation is a financial black hole. Add regulatory hurdles and process inefficiencies, and suddenly, your margins start shrinking fast.
CDMOs already have the infrastructure, tech, and talent in place. Instead of sinking millions into CAPEX-heavy operations, outsourcing lets you pay for only what you need, when you need it.
You get access to cutting-edge production methods without the headache of constantly upgrading your own facilities.
Phase-Appropriate Scaling
Scaling drug production isn’t a simple “more is better” equation. A process that works at 5 grams can fall apart at 500 grams if the right adjustments aren’t made.
The best CDMOs take a phase-appropriate approach, optimizing production at every stage—from preclinical trials to full-scale GMP manufacturing.
For example, medicinal chemistry often relies on solvents that become safety risks at higher volumes. CDMOs proactively swap out hazardous materials and refine conditions to improve efficiency without compromising quality.
Whether it’s reducing catalyst load, switching solvents, or modifying reaction parameters, these experts’ future-proof your process to ensure a seamless transition from lab to large-scale production.
More Sustainability
Many in-house processes can be waste-heavy, relying on resource-intensive methods that drive up costs and environmental impact. CDMOs leverage advanced bioreactors, continuous flow technology, and AI-driven process control to cut waste without sacrificing output.
Leading CDMOs are actively phasing out toxic, outdated solvents in favor of safer, eco-friendly alternatives that meet sustainability goals while improving yield.
Less waste means fewer purification steps and a streamlined production process. In a world where sustainability is becoming a competitive advantage, having a CDMO that prioritizes efficiency, and eco-conscious production can be a game-changer.
Choosing the Right CDMO: Key Considerations for 2025
Transparency in Pricing Models
While some CDMOs offer attractive base rates, unexpected costs for process adjustments, regulatory filings, or tech transfers can add up fast.
That is why you need full pricing transparency before signing any contract—not just for today but for every phase of development.
Look for CDMOs that offer clear cost breakdowns upfront. The best partners will map out potential cost escalations, helping you budget smarter. The only surprise you want in drug development is a breakthrough discovery—not a bloated invoice.
Regulatory Track Record
A CDMO can have the best tech in the world, but if they can’t navigate regulatory minefields, they’re a liability. FDA warning letters, compliance delays, and inconsistent documentation can derail your drug launch before it even gets off the ground.
Make sure to get answers to these two questions for separating the pros from the amateurs:
- Look at their past approvals. Have they successfully navigated multiple regulatory bodies (FDA, EMA, PMDA, etc.)?
- Do they have a history of seamless tech transfers without compliance hiccups?
A True Growth Partner
Some CDMOs will churn out your batches and call it a day. The best ones? They think long-term—aligning their success with yours.
How do you know if a CDMO is invested in your growth? Look for:
- Proactive problem-solving—Do they suggest improvements, or just take orders?
- Flexibility in scale-up—Can they handle a small-batch clinical trial today and full-scale commercial production tomorrow?
- Technology-forward approach—Are they keeping up with AI, automation, and next-gen manufacturing trends?
The right CDMO isn’t just an outsourcing solution. They’re your competitive advantage.
Making the Right CDMO Decisions in 2025
The industry’s top players aren’t going solo—they’re partnering with CDMOs that bring precision, expertise, and agility to the process.
In an era where regulatory landscapes shift overnight and efficiency defines market dominance, the right CDMO is becoming a necessity with each passing day.
With over 40 years of expertise and 300+ API processes under its belt, Neuland Labs has mastered the science of turning complex chemistry into commercial success. Neuland is the partner that 500+ biotech and pharma companies trust to accelerate drug development without sacrificing quality.
If you’re looking for a CDMO that delivers precision, efficiency, and speed, Neuland Labs is ready to help. Contact us today to see how we can bring your drug to market faster and without operational headaches.
FAQs
|
|
|
|