What Is Clinical Manufacturing? A Guide to Processes, Regulations, and Outsourcing
Global drug development is accelerating, but behind every clinical trial lies a hidden challenge: how to manufacture investigational new drugs (IND) at scale, compliantly, and on time. That’s where clinical manufacturing comes in.
This guide provides an industry-level overview of clinical manufacturing – explaining what it is, outlining key processes and compliance requirements, and discussing how partnerships with Contract Development and Manufacturing Organizations (CDMOs) can facilitate success.
What Is Clinical Manufacturing?
To put it simply, clinical manufacturing provides the investigational medicinal product that will be tested in humans for safety and efficacy during trials.
This means producing clinical batches of the drug candidate under controlled conditions before it is approved for mass production. These batches may include various dosage forms and often need to be modified or optimized between trial phases.
Because the goal is to support research, not to supply a commercial market (yet), the drug manufacturing process emphasizes agility, speed, and precision over high-volume output.
Key Differences from Commercial Drug Manufacturing
Clinical trial batches are generally smaller and might even be unique from batch to batch as the formulation is refined, whereas commercial manufacturing aims for consistent, identical batches at large scale.
Additionally, clinical supply chains can be more complex – for example, assembling patient kits with components from multiple sources (sometimes across continents) and ensuring cold chain storage for sensitive biologic drugs throughout distribution.
All of these factors make clinical manufacturing a distinct discipline within drug development, requiring careful planning and robust quality oversight.
Key Processes and Considerations in Clinical Manufacturing
Before clinical trial manufacturing, a detailed manufacturing plan and timeline are established. This plan aligns drug development milestones with manufacturing and trial needs.
Usually, the timeline is set before filing an IND or similar dossier to ensure drug supplies are ready when trials start. Experienced CDMOs help companies set realistic timelines and development plans.

Quality Control and Logistics
Each clinical batch undergoes rigorous testing for potency, purity, sterility (if applicable), and stability before release for trials.
Stability studies ensure it remains stable during the trial. Logistics are crucial — materials must be shipped worldwide to dozens or hundreds of trial sites.
Maintaining the supply chain is challenging, especially for biologics that require refrigeration or freezing. Any break in this chain can make the drug ineffective and jeopardize the trial, so companies invest heavily in logistics planning and contingency measures.
Ensuring Consistency
Because methods may change during development, ensuring consistency between batches is a challenge.
Nonetheless, consistency is paramount for trial integrity – if each patient dose is not made to a high and reproducible standard, trial results could be invalid. Each batch needs review and testing to ensure it meets specifications and changes were vetted.
Industry Best Practices
To mitigate risks in clinical production, regulators like the FDA have recommended several best practices for handling early-phase manufacturing. These include:
- Using disposable equipment and single-use process components to reduce cleaning requirements and risk of cross-contamination
- Using pre-sterilized containers and materials, which eliminates the need for dedicated cleaning equipment and simplifies setup
- Employing closed-system processing so that the drug is not exposed to the environment during manufacturing
- Leveraging qualified cGMP facilities or contract manufacturers (shared or external) for production and testing, rather than attempting the process in non-compliant labs
Regulatory Compliance in Clinical Manufacturing
Compliance with standards is vital in clinical manufacturing. All drugs for human use, including experimental ones, must follow current Good Manufacturing Practice (cGMP) guidelines set by regulators.
These guidelines ensure products are consistently made to quality standards, reducing risks beyond final testing. This requires clinical facilities to have quality systems covering personnel training, hygiene, equipment calibration, maintenance, and detailed documentation.
Regulations recognize the developmental nature of early trials and permit flexibility. In the U.S., FDA’s cGMP regulations for drugs are in Parts 210 and 211. Investigational drugs for Phase I are exempt from full Part 210/211 requirements; however, by Phase II and III, these regulations apply fully.
The FDA also recommends GMP practices in guidance to ensure safety during early development. Similarly, in the EU, the EMA requires medicines used in clinical trial manufacturing to meet GMP standards. The EMA inspects manufacturing sites and mandates that trial medicines be of consistent high quality and fit for use.
Any manufacturer worldwide must comply with EU GMP if their supplies are used in European trials. Non-compliance can cause delays or regulatory actions, emphasizing the importance of quality from the outset.
Partnering with CDMOs for Clinical Manufacturing
A strong Contract Development and Manufacturing Organization (CDMO) partnership allows a drug developer to accelerate the clinical batch manufacturing and focus on science and strategy while the CDMO manages the heavy lifting of production.
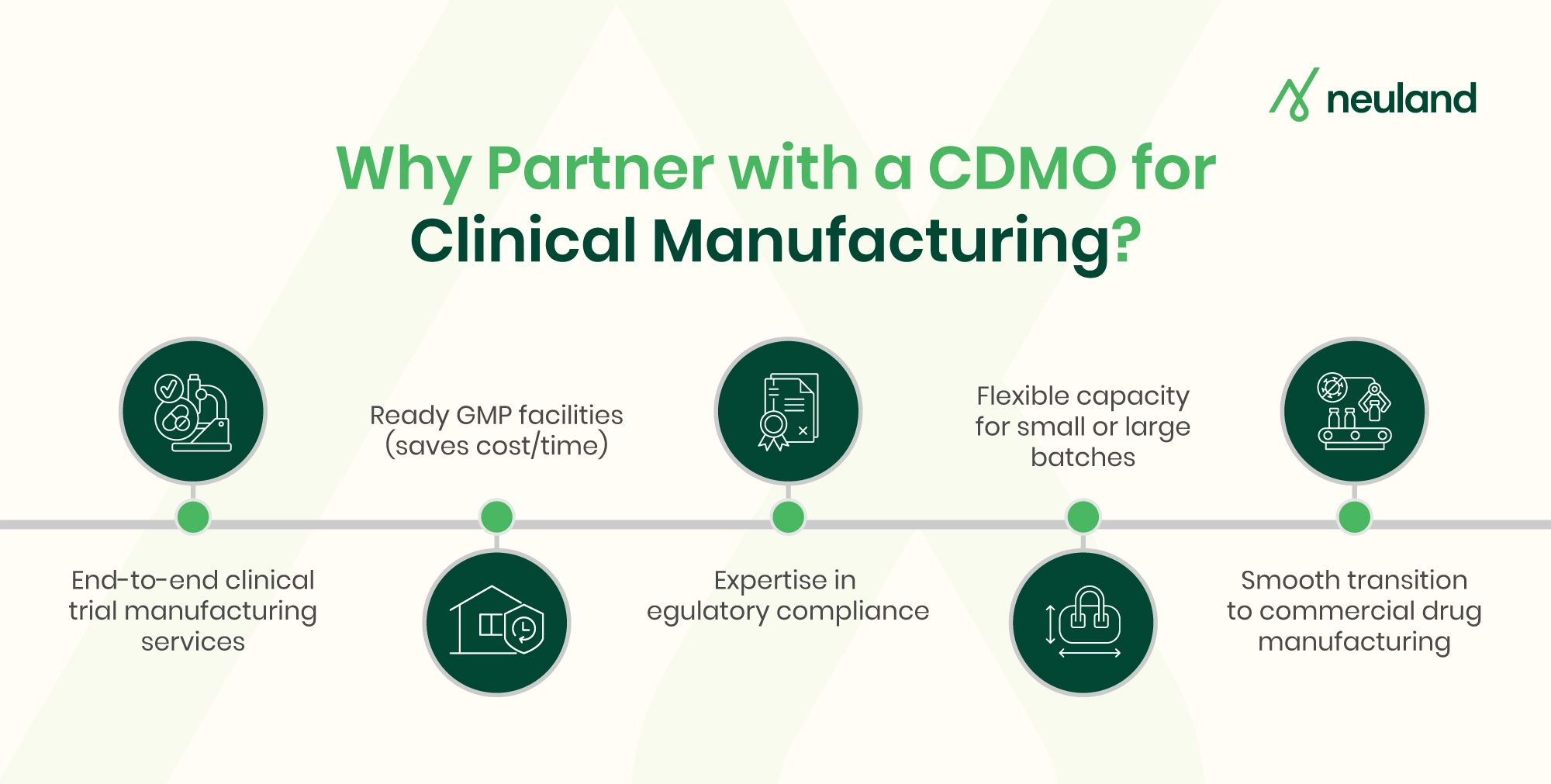
Leveraging an experienced contract manufacturer can shorten timelines to get investigational drugs into trials and ultimately get new therapies to market sooner. It also provides peace of mind that compliance and quality are being maintained by experts at every step.
For founders and clinical operations leaders, choosing the CDMO with a proven track record, relevant technical capabilities, and a collaborative approach can be a game-changer in navigating the complex journey of clinical manufacturing.
Clinical Manufacturing: Setting the Foundation for Success
Clinical manufacturing is key to drug development, turning lab breakthroughs into trial products. Understanding the process, adhering to quality standards, and partnering with CDMOs help de-risk this phase. A strong strategy ensures safe, efficient production, bridging promising candidates to therapies for patients.
At Neuland Labs, we understand the complexities of clinical manufacturing. As a global CDMO specializing in small molecules and peptides, we provide tailored solutions to help clients navigate early development through to commercialization.
With 4 decades of expertise, state-of-the-art facilities, and a focus on regulatory excellence, Neuland Labs delivers reliable clinical trial manufacturing services that ensure quality and speed-to-market.
If you plan on using a specialty CDMO for your clinical manufacturing needs, consider Neuland Labs. Our goal is simple: to help innovators transform promising molecules into life-changing therapies. Contact us today to get started.
FAQs
|
|
|
|