A CDMO’s Guide to Process Development & Scale-Up
Turning a new laboratory discovery into a successful medicine involves many complex steps, and one crucial step is process development and scale-up.
This is the phase where a promising molecule’s journey shifts from small-scale experiments to reproducible large-scale manufacturing – all while ensuring that every batch meets strict quality, safety, and efficacy standards.
For pharmaceutical companies, this transition is challenging; for a Contract Development and Manufacturing Organization (CDMO) like us, it’s where we excel. We bridge the gap between drug research and full-scale production by focusing on robust process development and methodical scale-up.
In this guide, we outline how CDMOs manage process development and scale-up from early development through commercial manufacturing, ensuring risks are mitigated, costs are controlled, and quality and compliance are built in.
What is Process Development in Pharma?
Process development in pharmaceutical industry settings involves creating efficient, cost-effective, and regulatory-compliant production methods while maintaining high product quality. It includes selecting raw materials, setting up equipment, optimizing reaction steps, and establishing in-process controls.
In essence, it turns a lab concept into a reproducible, scalable process by ensuring each step is efficient, reliable, and can be scaled up without issues. This is a multi-disciplinary effort: chemists, engineers, and manufacturing experts work together to test conditions and implement solutions that will work on a larger scale.
The result of effective chemistry and process development consistently produces the Active Pharmaceutical Ingredient (API) and final drug product at the required quality and yield. Laying this groundwork not only ensures product consistency but also improves efficiency – often reducing waste, boosting yields, and speeding time-to-market.
It also ensures the drug’s critical quality attributes (CQAs) are met batch after batch by tightly controlling key parameters.
Stages of Scale-Up From Lab Bench to Production
Process development and scale-up are about ensuring quality, consistency, and compliance across every stage. Each phase builds upon the last to reduce risks and optimize performance for commercial manufacturing.
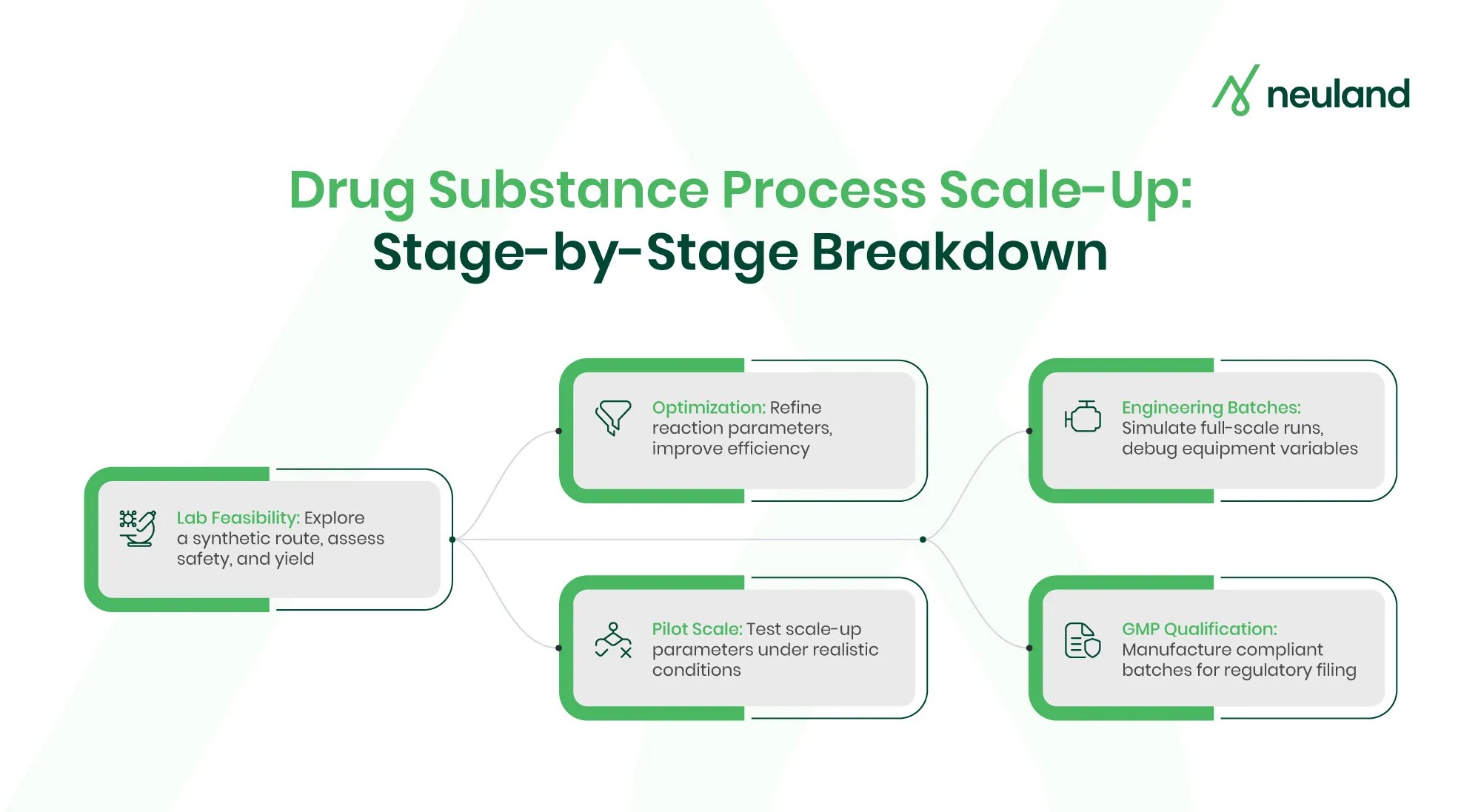
Key stages include:
- Route Scouting & Lab Feasibility
Small-scale batches are developed to evaluate reaction pathways, raw material availability, and process risks. This is where process safety and yield considerations begin. - Process Optimization
Once feasibility is confirmed, process parameters (temperature, pH, solvents, and catalysts) are optimized to improve yield and cost-efficiency. - Pilot Scale Demonstration
The process is scaled to tens of liters, mimicking plant-scale conditions. Critical process parameters (CPPs) and control strategies are fine-tuned. - Engineering & Scale-Up Batches
Engineering runs are conducted at manufacturing scale to test equipment fit, heat transfer, and mixing behavior—common pitfalls in tech transfer. - GMP Qualification Batches
These batches are produced under cGMP conditions and used for regulatory submissions (e.g., IND/NDA). Data from these runs supports full validation and filing.
Challenges in Scaling Up and Process Optimization Strategies

API scale-up from laboratory to manufacturing scale presents a host of challenges. Small-scale processes often need adjustments to work on a larger scale. Factors such as mixing efficiency, heat removal, and reaction kinetics can change when volumes increase, sometimes leading to lower yields or new impurities.
Such issues underscore why process optimization strategies are essential. Without proactive optimization, companies risk batch failures, quality deviations, or costly production delays.
To mitigate these risks, developers employ several techniques during process development and scale-up. One approach is to apply Quality by Design (QbD) principles – using design of experiments (DoE) to identify critical process parameters and define their acceptable ranges, ensuring the process stays robust as it scales.
Process safety is also a crucial aspect of scale-up. Certain steps that are harmless in the lab may pose hazards at the production scale, for example, generating much more heat or pressure.
In one case, our team eliminated a hazardous reagent and improved the overall process yields by 3-fold during scale-up by modifying the process chemistry. This exemplifies how smart development work can improve both safety and efficiency, reducing manufacturing risks and costs.
The CDMO’s Role in Process Development and Manufacturing
A capable CDMO plays a pivotal role in ensuring seamless process development and scale-up for drug sponsors. As a project moves from research into development, partnering with a CDMO brings specialized expertise, infrastructure, and experience to bear.
One major advantage of leveraging CDMO services is risk reduction and speed. For companies without extensive in-house development facilities, a CDMO’s process development expertise and ready infrastructure fill the gap.
By entrusting CDMO process development experts with scale-up, drug innovators can focus on their core discovery and clinical efforts, confident that the transition to manufacturing is in capable hands.
Achieving API Scale-Up The Neuland Way
Effective process development and scale-up are the cornerstones of turning a new molecule into a viable treatment.
These principles form the core of how we operate at Neuland Labs. In all our pharma process development projects, we emphasize these principles. With over 40 years of experience in developing complex small molecules and peptides, we have guided countless projects from early development through successful commercial manufacturing.
Achieving success in this domain requires both scientific rigor and practical manufacturing know-how – and this is how we do it at Neuland. We combine deep expertise, state-of-the-art facilities, and an unwavering focus on quality and efficiency at every step.
By working closely with our partners and leaving no detail overlooked, we ensure that scale-up, tech transfer, and production are executed right.
Check out how Neuland supports process development and scale-up, which leads to life-changing medicines reaching patients faster and more reliably.
FAQs
|
|
|
|