Why Pharma Giants Are Outsourcing Drug Manufacturing to CDMOs
Pharmaceutical companies face a crucial decision: build in-house development facilities or outsource manufacturing to scale production.
Building in-house facilities involves long-term resource commitments and ongoing maintenance. This burden often slows down innovation and diverts focus from critical R&D.
Therefore, pharmaceutical companies are now turning to external partners who already have the expertise, cutting-edge equipment, and regulatory know-how to bring drugs to market faster and more efficiently.
Enter Contract Development and Manufacturing Organizations (CDMOs)—the backstage crew making sure new drugs don’t get stuck in the development phase.
These partners help pharma companies overcome some of the biggest hurdles in drug manufacturing: lack of capacity, high production costs, and the ever-present risk of delays.
And the industry is catching on.
The global pharmaceutical CDMO market is projected to hit $315 billion by 2034, growing at a 7.24% annual rate. With the rising demand for pharmaceutical products, CDMOs are becoming key to the drug development and manufacturing ecosystem.
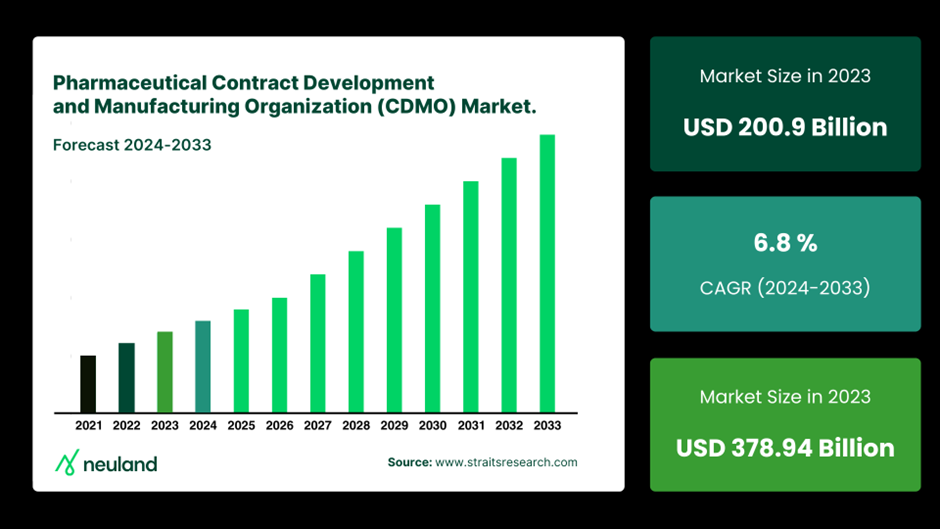
But what exactly do CDMOs do, and how do they add value to pharmaceutical manufacturing? Let’s break it down.
Why Contract-Based Pharma Manufacturing is Booming
The rise in contract-based pharma manufacturing is driven by the complexity of drug development and the need for speed.
Advanced manufacturing is in demand for biologics, personalized medicine, and specialized therapies.
CDMOs meet these needs, integrating drug development stages with manufacturing from preclinical trials to commercial supply.
Their services ensure smooth, compliant, and cost-effective drug development, backed by extensive regulatory expertise.
From IND/NDA submissions to compliance with global regulations (FDA, EMA, and other regional governing bodies), CDMOs offer valuable support in navigating this maze, reducing the risk of costly delays or issues down the line.
Contractual Services for Pharma Manufacturing
CDMOs offer comprehensive services throughout the drug manufacturing process.
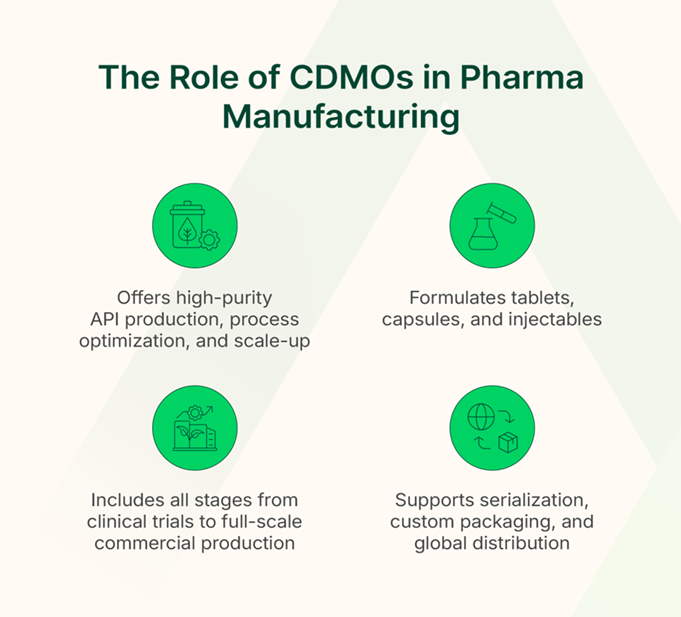
Drug Substance Manufacturing (API Production)
The active pharmaceutical ingredient (API) is crucial for the therapeutic effect of drugs. CDMOs offer various services in API production, including:
Small-Molecule API Synthesis
CDMOs optimize complex synthesis processes to maximize yield, reduce impurities, and ensure batch consistency. They handle volatile or hazardous compounds that may not be managed in-house.
High-Potency API (HPAPI) Manufacturing
Focused on potent therapies for oncology and autoimmune diseases, HPAPIs need specialized facilities to prevent contamination and protect workers. CDMOs use high-containment suites with negative pressure rooms, isolators, and automation for safe production.
Process Optimization & Scale-Up
CDMOs refine reaction conditions, reduce waste, and improve efficiency from gram to metric ton quantities. Using computational modeling, real-time monitoring, and analytics, they ensure optimized API synthesis for commercial demand without quality loss.
Continuous Manufacturing & Green Chemistry Initiatives
CDMOs are moving from traditional batch manufacturing to continuous processes, reducing downtime, enhancing quality control, and speeding up production. Green chemistry focuses on safer solvents, cutting energy use, and minimizing hazardous byproducts for more sustainable drug manufacturing.
Peptide API Manufacturing
Synthetic peptides are driving advancements in next-generation drugs, diagnostics, and antibody production.
These short chains of amino acids are utilized in various applications, from diabetes treatments to cancer therapies. However, large-scale production poses significant challenges. Specialized CDMOs, such as Neuland Labs, offer extensive expertise in:
- Solid-phase peptide synthesis (SPPS) for expedited and automated production
- Solution-phase synthesis for high-yield bulk manufacturing
- Hybrid technologies to address complex and multi-chain peptides
Drug Product Manufacturing
Once the API is prepared, the focus shifts to formulating it into an administrable drug. Whether it’s a pill, injection, or powder, CDMOs manage the intricate process of drug product manufacturing with precision and efficiency.
Oral Solid Dosage (OSD) Manufacturing
CDMOs meticulously adjust parameters such as compression force to ensure pills maintain appropriate consistency and coating technology to ensure proper dissolution within the body.
They also handle specialized formulations, including controlled-release tablets, multi-layer tablets, and orally disintegrating tablets (ODTs) for patients who experience swallowing difficulties. Additionally, CDMOs possess advanced granulation, blending, and tablet compression technologies capable of producing millions of doses efficiently.
Sterile & Injectable Manufacturing
Injectable drugs require near-zero contamination levels. A single microbial error could result in a global recall and substantial financial losses.
CDMOs operate high-grade cleanrooms with aseptic filling lines for vials, ampoules, and pre-filled syringes, ensuring zero microbial contamination.
They also manage lyophilization (freeze-drying) processes that extend the shelf life of fragile biologics. With the increasing prevalence of self-administered injectables, CDMOs assist in designing auto-injectors and pre-filled pens to facilitate safer and more convenient at-home dosing for patients
Clinical & Commercial-Scale Manufacturing
CDMOs ensure that every batch used in clinical research is produced from reliable regulatory starting materials (RSMs) to avoid inconsistencies that could affect drug approval.
With strict quality controls in place, they manufacture APIs for Phase I through Phase III trials, maintaining high standards throughout early-stage development to commercial launch.
CDMOs collaborate with pharmaceutical companies to customize production strategies based on clinical timelines, regulatory requirements, and scale-up potential.
They ensure that API production meets the demands of clinical research while staying flexible enough to adjust for formulation changes, dosage refinements, or evolving trial needs.
When it’s time for technology transfer, a CDMO ensures that methods, formulations, and production details are seamlessly transitioned to full-scale manufacturing—whether within their own facilities or to another global production site.
Packaging & Supply Chain Services
CDMOs assist pharmaceutical companies with packaging and ensuring the drug reaches the market efficiently and safely. Services include:
- Serialization & track-and-trace compliance: CDMOs integrate track-and-trace technology into packaging by assigning unique barcodes or RFID tags to every product, ensuring authenticity and supply chain transparency.
- Custom packaging solutions: They provide tailored packaging solutions, including child-resistant, tamper-proof, or single-dose options such as blister packs, bottles, pre-filled syringes, and sterile vials, all while ensuring stability, shelf life, and patient ease of use.
- Cold-chain logistics & distribution: For drugs such as biologics and vaccines that require strict temperature control, CDMOs manage cold-chain logistics using specialized freezers, temperature-controlled transit, and real-time monitoring to prevent spoilage and ensure regulatory compliance from factory to final destination.
How to Choose the Right Contract Manufacturing Services for Your Pharmaceutical
Selecting the right pharma contract manufacturing partner is crucial.
Top CDMOs offer API synthesis, drug product manufacturing, clinical and commercial production, and robust packaging and supply chain solutions. Ensure your partner meets these criteria and can adapt to industry changes.
Neuland Labs, with 40 years of experience, provides comprehensive drug development services from early-stage R&D to full-scale commercial manufacturing. They excel in process development, optimization, analytical testing, and regulatory support, working closely with pharmaceutical innovators. Contact us today to learn how Neuland can assist your drug development journey
FAQs
|
|
|
|