Custom Peptide Synthesis: Choosing the Right Method for Your Sequence
Custom peptide synthesis is a cornerstone of modern biotechnology and pharmaceutical research, enabling the production of specific peptide sequences on demand. However, not all peptides are made the same way.
There are different synthetic methodologies, each with its own advantages and limitations. The two primary peptide synthesis methods are solid-phase peptide synthesis (SPPS) and liquid-phase peptide synthesis (LPPS).
In this article, we provide an authoritative overview of SPPS vs. LPPS, including their pros and cons, and offer guidance on selecting the optimal approach for your specific peptide needs.
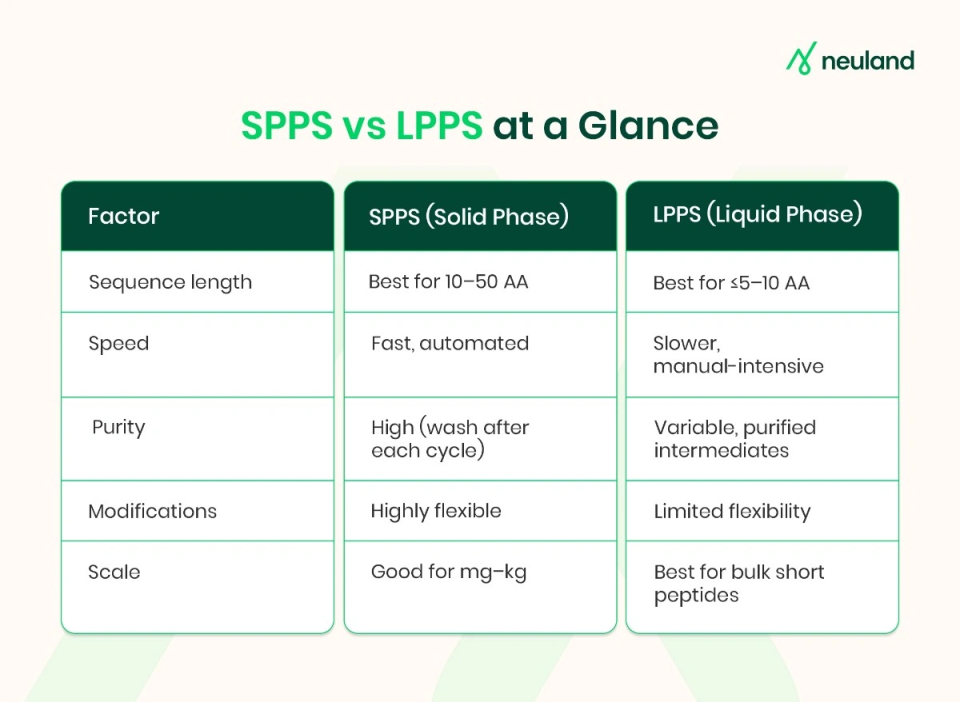
Solid-Phase Peptide Synthesis (SPPS)
Solid-phase peptide synthesis is the most widely used technique for custom peptide synthesis today. In SPPS, the peptide chain is assembled stepwise on an inert solid resin support.
Each amino acid is added sequentially to the growing chain, with washing and deprotection steps in between, until the full sequence is built. This method has become prevalent because it dramatically reduces the number of isolation steps required for long peptide chains, offering high efficiency and yield even for complex sequences.
Modern SPPS can be automated and even microwave-assisted, allowing precise control and improved synthesis of longer peptides.
Key Advantages of SPPS
- Efficiency & Speed: Faster than solution methods, especially for longer peptides. Excess reagents drive reactions to completion, with simple washes removing by-products.
- High Purity: Anchoring peptides to solid supports allows repeated wash cycles, minimizing impurities and suppressing side reactions.
- Automation & Throughput: Easily automated with peptide synthesizers, enabling parallel, high-throughput peptide production.
- Flexibility: Supports peptide modifications like D-amino acids, PEGylation, fluorescent tags, disulfides, and cyclic structures, making it ideal for complex peptides.
Downsides: SPPS is resource-intensive, consuming large volumes of solvents and reagents, raising costs and environmental concerns. Extremely long peptides also suffer from reduced yields and purification challenges, often requiring hybrid strategies.
Liquid-Phase Peptide Synthesis (LPPS)
Liquid-phase peptide synthesis is the classical solution-based approach, where each amino acid coupling requires isolation and purification before the next step. This makes it less practical for long chains, as yields drop with repeated purifications.
Today, LPPS is mainly used for short peptides (2–10 amino acids), fragments, or building blocks. Its key advantage is scalability and solvent economy, since reactions occur in solution with less reagent excess, and intermediates can sometimes be crystallized for easier purification.
Hybrid Strategies and Fragment Condensation
SPPS and LPPS are often combined to balance speed and practicality. In these hybrid approaches (fragment condensation or convergent synthesis), medium-sized fragments are built by SPPS and then linked in solution.
This method avoids the yield drop of long SPPS chains and the inefficiency of pure LPPS. Techniques like native chemical ligation (NCL) allow assembly of peptides well over 50 amino acids, even up to 100+.
By stitching together high-purity fragments, hybrid strategies make complex sequences achievable, playing a critical role in peptide API manufacturing when targets exceed SPPS’s linear limits.
Key Considerations for Choosing a Custom Peptide Synthesis Method
When deciding between SPPS, LPPS, or a combined strategy for a custom peptide, consider the following factors:
- Sequence Length: Perhaps the most important factor. For short peptides, LPPS can be a viable and cost-effective method. For medium-length peptides, SPPS is usually the method of choice due to its reliability in assembling longer chains. For very long peptides, especially those approaching protein size, plan on using fragment condensation or other advanced techniques to ensure acceptable yields.
- Complexity and Modifications: If your sequence includes unusual features – non-standard amino acids, multiple disulfide bonds, cyclic constraints, or post-translational modifications – SPPS will typically offer more flexibility. Solid-phase methods allow incorporation of a wide range of building blocks and on-resin transformations that custom peptide synthesis services routinely offer. LPPS, while not impossible for modified peptides, often complicates protecting group chemistry and yields.
- Required Quantity (Scale): Many peptide drug APIs on the market are manufactured by large-scale SPPS with batch reactors and even continuous-flow synthesizers. However, the cost of reagents and solvent waste in SPPS grows with scale. If the peptide is relatively simple and needed in huge quantities, a well-optimized LPPS process might reduce raw material costs and waste generation. In some cases, companies develop an SPPS route for initial R&D and then investigate a solution-phase or hybrid route for economic large-scale production. Engaging a peptide manufacturing partner with experience in both methods can help identify the most scalable solution.
- Timeline and Budget: For most research-scale projects, SPPS is faster from start to finish. Automated synthesizers can crank out a peptide in a day or two, whereas a manual LPPS of the same length could take much longer. If time is critical, SPPS (or ordering from a custom peptide synthesis service that uses SPPS) is usually indicated. Budget-wise, SPPS’s cost grows with peptide length (resin and reagent costs), but for small quantities this is usually acceptable. LPPS might save money on reagents for short peptides or very large batches, but the trade-off is higher labor/process costs. It’s worth discussing with your peptide supplier which approach minimizes cost for your specific case – sometimes a slightly more expensive synthesis method can save downstream purification costs or time.
Peptide Manufacturing and Regulatory Considerations
In moving from research to development, synthesis must align with GMP standards. Both SPPS and LPPS are used in peptide manufacturing, though SPPS is often preferred for its automation, reproducibility, and proven success in approved drugs like leuprolide.
LPPS or hybrid approaches can be advantageous for greener, large-scale processes, where crystallization or solution-phase routes reduce dependence on HPLC bottlenecks. Facility capabilities often determine the choice—some specialize in large SPPS reactors, others in solution chemistry.
Regardless of method, regulatory compliance requires purity, consistency, and clear analytical characterization. The best practice is to optimize for quality in development, then scale with process refinements.
Partnering with an experienced CDMO offering custom peptide manufacturing ensures the right balance of technical feasibility and compliance for peptide APIs.
Partnering with an Experienced Custom Peptide Synthesis Partner
There is no single “best” method in custom peptide synthesis—SPPS offers speed and flexibility, LPPS supports short or scalable runs, and hybrid strategies extend reach to complex sequences. The right choice depends on length, complexity, and manufacturing scale.
For biotechnology and pharmaceutical projects, early consultation with an experienced CDMO is invaluable. They can assess your sequence and guide you toward the optimal route, ensuring high-quality, compliant peptides for development or commercialization.
Neuland Labs, a global CDMO specializing in small molecules and peptides, exemplifies this expertise. With four decades of process chemistry experience and capabilities in both SPPS and LPPS, Neuland supports clients worldwide in selecting, scaling, and delivering peptide APIs with confidence. Connect with us today.
FAQs
|
|
|
|