Analytical Method Validation: Key Parameters & Common Challenges
Analytical method validation (AMV) is a fundamental process to ensure every test method produces reliable and accurate data. This rigorous process is mandated by regulators worldwide as part of method validation in pharma to safeguard drug quality and patient safety.
In practice, analytical method development and validation go hand-in-hand: scientists first develop and optimize a method, then validate it to confirm it meets all regulatory criteria for performance.
Whether validating an assay for a small-molecule API’s potency or an HPLC impurity test for a therapeutic peptide, the goal is the same – to ensure the method is fit for purpose and compliant with global standards.
Key Parameters of Analytical Method Validation
A successful analytical method validation evaluates several critical performance characteristics to establish that the method will consistently produce trustworthy results. The key parameters of analytical method validation include:
- Accuracy – The closeness of test results to the true value. Typically assessed by recovery studies (e.g., spiking known standards into samples) to ensure the method measures the analyte without bias.
- Precision – The degree of agreement (repeatability) among multiple measurements of the same sample. This includes intra-assay repeatability and intermediate precision (variation across different days, analysts, or labs) to confirm the method yields consistent results.
- Specificity – The ability to unequivocally measure the target analyte in the presence of other components like impurities, degradants, or matrix ingredients. A specific method can distinguish the analyte from everything else, which is crucial for stability-indicating methods.
- Limit of Detection (LOD) & Limit of Quantitation (LOQ) – The smallest amount of analyte that can be detected, and the lowest level that can be quantified with acceptable accuracy and precision. These parameters establish the method’s sensitivity, important for trace impurities or potent drugs.
- Linearity & Range – The method’s ability to elicit results directly proportional to analyte concentration (linearity), and the span over which this linear relationship holds with suitable accuracy and precision (range). This ensures the method can reliably quantify the analyte from the lowest to the highest required concentrations.
All these analytical method validation parameters must fall within predefined acceptance criteria based on regulatory method validation guidelines (e.g., ICH) to confirm the method’s fitness for use.
In essence, these AMV parameters collectively demonstrate that the analytical procedure will produce quality data day in and day out.
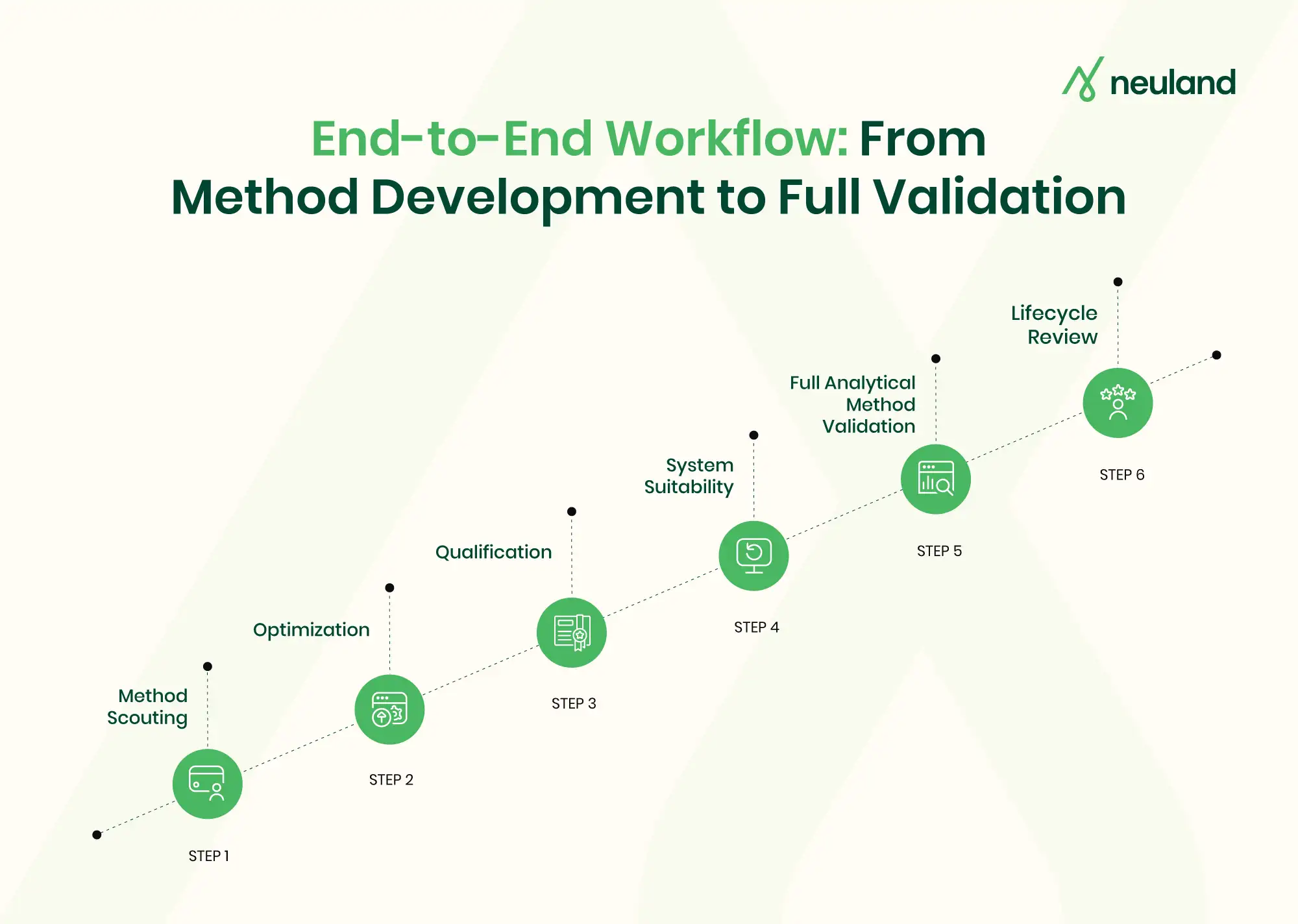
Regulatory Method Validation Guidelines and Expectations
Regulatory agencies worldwide have well-defined expectations for analytical method validation in pharma, and these expectations are largely harmonized globally.
The cornerstone of global guidance is the International Council for Harmonisation (ICH) guideline Q2, which was recently revised as ICH Q2(R2) to modernize the approach to validation.
ICH Q2(R2) Guidelines
ICH Q2 is the internationally accepted guideline defining how to validate analytical procedures. The latest revision of ICH Q2(R2) (finalized in late 2023) updates the previous Q2(R1) by expanding the scope to modern techniques and emphasizing a more scientific, risk-based approach.
Under ICH Q2(R2), method validation is not just a box-checking exercise but part of an analytical procedure’s lifecycle – starting from method development through to routine use and changes over time. The guideline outlines the core validation characteristics (accuracy, precision, specificity, LOD, LOQ, linearity, range, robustness, etc.) that must be evaluated to demonstrate a method’s fitness for intended use.
Importantly, ICH Q2(R2) incorporates principles aligned with Quality by Design and pairs with the new ICH Q14 (Analytical Procedure Development) guideline. This means there is greater emphasis on defining an Analytical Target Profile (ATP) at the outset and using risk management to design robust methods.
FDA, USP, and Other Global Guidelines
As a founding member of ICH, the U.S. FDA adopts ICH’s method validation standards, making them effectively part of FDA requirements for drug submissions.
In practice, complying with ICH Q2(R2) goes a long way toward meeting FDA expectations for analytical procedures in INDs, NDAs, and ANDAs. The FDA’s own guidance aligns with the ICH principles, reinforcing that methods should be scientifically sound, suitable, and well-documented.
Likewise, the European Medicines Agency (EMA) and other regulators in ICH regions require that validation data accompany any quality control method used in pharmaceutical applications, following the ICH Q2 criteria.
Pharmacopeial standards also echo these requirements. For example, the United States Pharmacopeia USP <1225> “Validation of Compendial Procedures” outlines similar validation parameters and tests, ensuring even compendial (pharmacopoeial) methods meet the same high bar.
Other standards and agencies (such as ISO for laboratory accreditation or CLIA for clinical labs) have their own method validation or verification guidelines, but for drug development analytical method validation in pharma, ICH Q2 remains the global gold standard.
Common Challenges in Analytical Method Validation
Developing a rigorous, “phase-ready” analytical method – one that will smoothly support drug development from early clinical phases through commercialization – comes with several challenges.
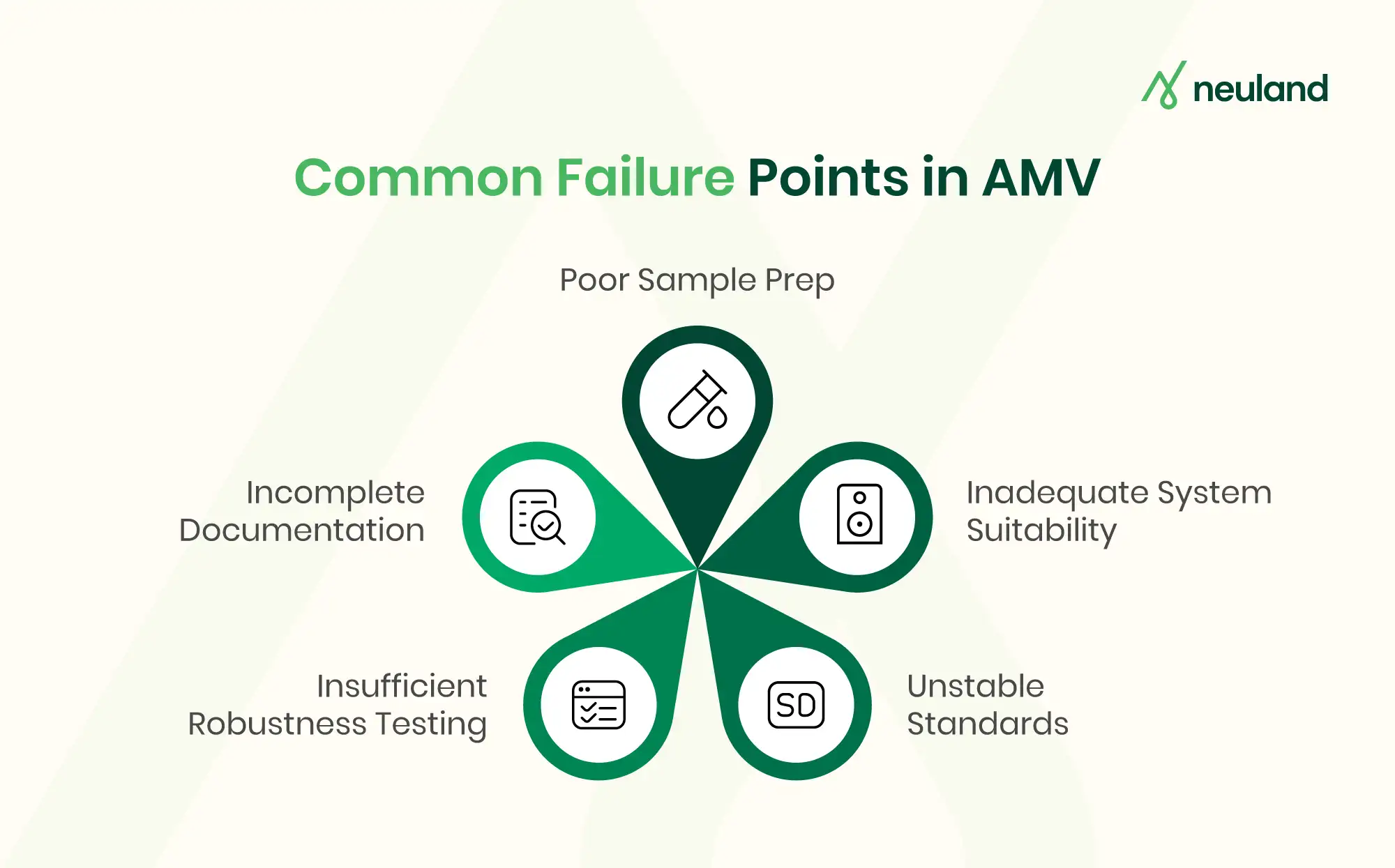
Below are some common challenges and pitfalls in method validation in pharma, especially pertinent to small molecule and peptide API analytics:
- Ensuring Specificity in Complex Samples: One frequent challenge is achieving adequate specificity. The method must distinguish the analyte from impurities, degradation products, excipients, or matrix components. Analytical development validation often requires trials with forced degradation samples and multiple columns or conditions to ensure specificity is robust. Without proper specificity, results become unreliable and can trigger regulatory concern.
- Sensitivity and Low-Level Quantitation: Analytical methods often need to detect and quantify trace-level impurities or low-dose analytes. Setting very low LOD/LOQ can be difficult due to instrument limitations or background noise. Analysts must optimize sample preparation and detection (sometimes using more sensitive techniques like LC-MS/MS) to reach the necessary sensitivity. Failing to achieve the required LOQ for a genotoxic impurity, for instance, could delay a drug program until the method is improved.
- Method Robustness and Transferability: A method that works well under one set of conditions might falter when minor variations occur or when transferred to another lab. Assessing robustness is therefore critical: during HPLC method validation, for instance, small changes in flow rate or solvent composition can cause noticeable shifts in retention times and potentially affect accuracy. If a method is too fragile, day-to-day variability in equipment or reagent quality could lead to out-of-specification results.
- Peptide-Specific Analytical Issues: Peptide drug substances share many validation considerations with small molecules but also pose unique challenges. Peptides can be unstable (prone to enzymatic or chemical degradation) and may adsorb to labware surfaces, causing analyte loss. They might also have solubility issues in standard solvents. Validating a peptide method often involves extra checks for stability-indicating capability and recovery from surfaces. Overlooking these nuances can result in inconsistent data.
- Regulatory Compliance & Phase-Appropriate Validation: Early in development (Phase I/II), not every validation test from ICH Q2 may be fully executed – a phase-appropriate approach might focus on essential parameters to confirm the method is scientifically sound and suitable for its purpose. However, by late-phase (Phase III) and for NDA submission, regulators expect a complete validation per ICH Q2 with all parameters and stress studies addressed. It can be challenging for teams to decide how much validation is “enough” at each stage – too little might risk data integrity, too much too soon could waste resources if the method or process changes. Clear method objectives and an understanding of regulatory expectations are crucial to navigate this.
Overcoming Analytical Method Validation Challenges With A Partner
While challenges such as achieving high specificity, sensitivity, and robustness can complicate the process, these can be overcome with sound scientific strategies and careful planning.
Additionally, global regulatory expectations demand consistency, so it is great to have a partner by your side to adequately work through the analytical method validation process.
As a global contract development and manufacturing organization (CDMO), specializing in small molecules and peptides, Neuland underscores the importance of rigorous method development and validation in our practice.
We operate three cGMP manufacturing facilities with a new reactor capacity of 1,174,000 liters and have an impressive track record, having supported 25 IND filings and 4 NDA filings to date.
Connect with our team today and let us ensure the analytical methods for your projects are phase-appropriate and aligned with the latest guidelines.
FAQs
|
|
|
|