
What Trends Are Transforming the CDMO Industry in 2025 and Beyond

Why Early Manufacturing Process Optimization is the Safest Path to Efficient Scale-Up

What Is a Boutique CDMO? Key Characteristics & Role in Pharma
Custom Peptide Synthesis: Choosing the Right Method for Your Sequence

Custom Peptide Synthesis Partner: Key Factors for Selecting the Best Fit

What Is Clinical Manufacturing? A Guide to Processes, Regulations, and Outsourcing

CDMO vs CMO vs CRO: Choosing the Right Outsourcing Partner in Drug Development

API Micronization in the Early vs. Late Stages of Drug Development: Goals and Challenges

From Milligrams to Kilograms: Scaling Up Synthetic Peptides

CDMO Market Outlook 2025–2030: Trends Shaping the Future of Pharma Outsourcing
Why a Customer-First Mindset Is the Key Driver of Innovation in CDMOs

Peptide Manufacturing at Commercial Scale: What to Expect from a Trusted CDMO Partner

Liquid Phase Peptide Synthesis: Guide to Methods, Use Cases, & Strategic Advantages

Peptide Conjugation - Common Conjugation Strategies

CDMO vs. CMO: Understanding the Outsourcing Models That Drive Drug Development

Peptide vs. Protein: Breaking Down 5 Essential Differences for Drug Makers

A Complete Guide to Commercial Manufacturing for Pharma Innovators

Your Guide to CDMO Services: What Leading Pharma Teams Rely On

Building a More Sustainable Supply Chain in Pharma

Why the CDMO Industry Matters More Than Ever in Drug Development

Small Molecule Drug Development: Process, Strengths, and CDMO Role

What Is Solid Phase Peptide Synthesis? Process, Uses & Benefits

Business Continuity Management—the Next Frontier in API Supply Chain Security

How CDMOs Help Biotech Advance and Navigate Drug Development and Manufacturing Complexities

The Ultimate Guide to Selecting a CDMO Partner

Understanding cGMP Compliance for Pharmaceutical Manufacturing Excellence

Why Pharma Giants Are Outsourcing Drug Manufacturing to CDMOs

All You Need to Know About Outsourcing Drug Manufacturing & Development to CDMO

5 Reasons Why More Pharma Companies Are Strengthening CDMO Relationships

Quality Risk Management in Pharmaceutical Industry

Neuland Announces New Vision and Values Statements

The Essential Role of Intellectual Property Expertise in a Pharmaceutical API Company

Achieving Drug Development Success: Inside Neuland’s Three-Pillar Project Management Strategy

From the Neuland Reading Room: Peptide Trends in 2024

Zero Liquid Discharge: A Pharma Industry Commitment to Environmental Sustainability

The Pharma Supply Chain: Reshaping Itself for The New Normal

Redefining Excellence: Neuland Unveils a Refreshed Brand Identity

From the Neuland Reading Room: A Check-in on 2024 Trends in the CDMO Industry

How to Ensure Effective Technology Transfer to CDMOs

Why Agility Matters When Choosing an API Contract Manufacturing Partner

API Particle Size Part 2: New & Emerging Technologies Impacting Particle Size

API Particle Size Part I: Overcoming the Challenges to Ensure Quality Pharmaceutical Products

Scaling-up Active Pharmaceutical Ingredient (API) Chemistry for Phase 3 Clinical Trials and Bulk Manufacturing for a Potentially Novel Treatment for Schizophrenia

R&D Expert Talks Pharma API Scale Optimization

The Crucial Role of Crystallization in Drug Substances Development

What Pharmacological Advantages Can Deuterated APIs Deliver?

Integrating Sustainability into Pharma Manufacturing

8 Ways Effective Supply Chain Management Can Minimize Pharmaceutical Supply Chain Challenges

Advancing a Cholesterol Lowering Drug at the Height of the Pandemic

ESG in Pharma: Building Sustainable Businesses

How Neuland’s Process Engineering Lab Supports Robust Design and Minimizes Risk

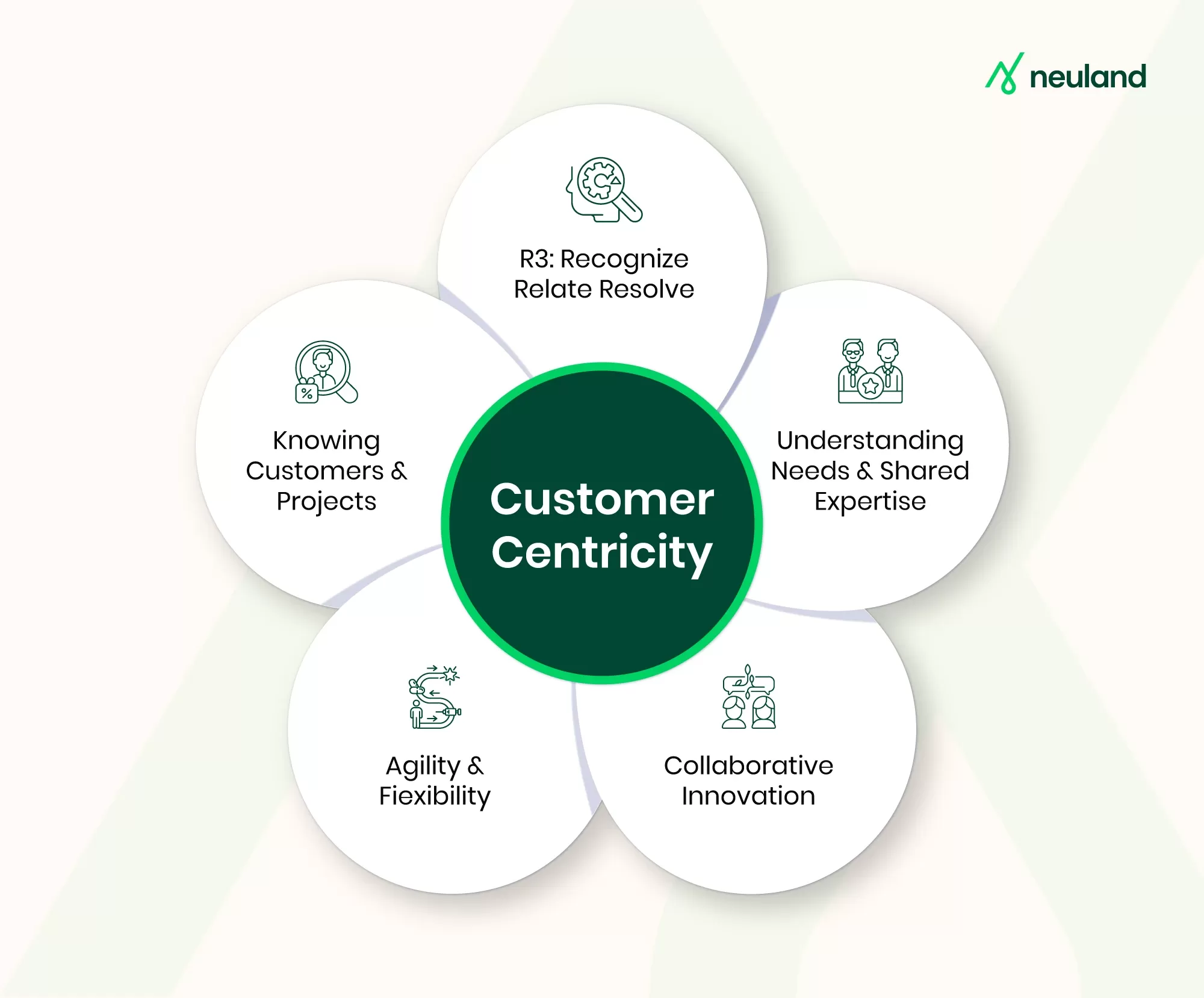
Putting Customers First: Customer Centricity in Pharma API Manufacturing

3 Key Elements of a Successful Hydrogenation Scale-Up

Why is Pharmaceutical Green Chemistry Use on the Rise?

Regulatory Starting Materials: How to Source, Analyze and Validate Suppliers (Part I)

Exploring Neuland’s Therapeutic Segments: Focus on CNS

Green Chemistry by Design (GCbD): The Earlier, The Better for Pharmaceutical APIs

Deuterated Drug Molecules: Perfecting the Gamechanger

What Does it Take to Become a Specialist in Late-Phase CMS?

API Production: Building a Process Safety Culture

Looking for an API Supplier? 5 Things to Consider (Updated)

Nitrosamine Impurities and How Companies are Addressing the Crisis

Successful Pharmaceutical Project Management

Complex Synthesis in Action: Achieving a Commercial-Scale 16 AA Peptide

A Guide to Sourcing Pharmaceutical Peptide APIs

Case Studies from Neuland’s Process Engineering Lab

Overcoming Challenges in Complex Peptide Purification

Welcome, Unit 3!

QbD and Evaluating ‘What-If’ Drug Manufacturing Scenarios

NCEs Versus Generics – Adjusting Project Tactics & Objectives to Maximize Success

Synthetic Route Scouting: Factors to Improve API Manufacturing

How to Select a Peptide Synthesis Technique

Leveraging QbD for API Scale Up

5 Common Challenges Scaling Up an API

How Process Engineering Techniques Result in Cost-Effective Products

API Manufacture of Deuterated Molecules

Advantages of a Pure-Play API Contract Manufacturer

APIs & Continuous Flow Manufacturing

Challenges with API Micronization Techniques for Use in Injectables and Medical Devices

Advances in the Synthesis of Peptide Therapeutics: Clear Advantages…and Some Barriers

Genotoxic Impurities – Increasing Vigilance, But Still Some Uncertainty

6 Properties of API Synthesis that Affect Yield, Delivery Date & Purity

Contract Drug Manufacturing R&D: from Route Scouting to Kilo Labs

The Benefits of Synthesis Scouting

Analytical Capabilities and Drug Quality – Key to Successful Pharma Outsourcing

The New Look of Neuland

Peptide Synthesis: Solution Phase, Solid Phase or Hybrid?

Neuland Labs Receives 2013 CMO Leadership Award for Regulatory Excellence
