8 Ways Effective Supply Chain Management Can Minimize Pharmaceutical Supply Chain Challenges
Posted on April 27, 2023 by Saharsh Davuluri
 Supply chain disruptions leading to drug shortages aren’t a new challenge. In fact, the FDA maintains a database of current shortages. But the problem appears to have become more pervasive over the last few years.
Supply chain disruptions leading to drug shortages aren’t a new challenge. In fact, the FDA maintains a database of current shortages. But the problem appears to have become more pervasive over the last few years.
According to the World Economic Forum multiple nations around the world are experiencing essential drug shortages. A survey conducted by the Pharmaceutical Group of the European Union noted medicine shortages in the 29 EU-member countries from mid-November 2022 through December 2022. Interestingly, 76% reported shortages that were worse than those experienced in 2021.
European nations are not alone. Hospitals in the US lack adequate supplies of liquid ibuprofen. Other drugs in high demand but short supply include Tamiflu, Amoxicillin, and Adderall. In Argentina, supply chain issues have inflated the cost of cancer medications by more than 50%, while Panama currently has the most expensive drug costs in Latin America.
All these issues are the direct result of breakdowns in pharmaceutical supply chains.
Effective supply chain management depends heavily on information- and data sharing up and down the entirety of the supply chain. Supply chain breakdowns, as seen during the pandemic, typically result from a lack of forethought, collaboration, or management in place to remedy the supply chain deficit. The impacts felt over the last several years have resulted in a tremendous shift in supply chain management approaches, with an emphasis on supply continuity.
As we’ve all now experienced, when supply chain management (SCM) is ineffective, the results are quickly felt by consumers…and have serious repercussions. Wallace J. Hopp, a University of Michigan professor, explains:
“Disruptions in medical product supply chains have greater implications than making people wait for a new television set. They have the potential to seriously compromise patient care.”
 Managing the Links of the Pharmaceutical Supply Chain
Managing the Links of the Pharmaceutical Supply Chain
Pharmaceutical supply chains include a sophisticated network of partners— suppliers, manufacturers, storage facilities, and distributors—all working to supply, produce, deliver, and sell quality drug products. These global systems involve numerous processes, individuals, policies and technologies, and their effective management is indispensable to the success of the pharmaceutical industry.
Mitigating supply chain risk demands continual vigilance in which every component and stage of the process is scrutinized and strategized.
It is not enough to merely identify risk. Companies must address potential issues with prompt and calculated actions and remain constantly alert to supplier issues.
Logistics challenges have been magnified by increasingly complex drug products. For example, many compounds – including those associated with mRNA vaccines – require refrigeration. The resulting cold chain logistics necessary to maintain product integrity have led to an increase in the risk of nonconformity.
Thankfully, Neuland’s commercial cold chain shipments have suffered no temperature outages, and every product for which we are responsible has remained in conformance. Our cold chain logistics success is directly attributed to having built reliable collaborations.
Maintaining Supply Chain Quality and Timeliness
The last few years have been a challenge for companies operating on a global scale. We’re proud of our successes despite the broader, global impacts felt across supply chains. Robust planning, strong communications, and a “proactive preparedness” approach have proven critical to consistent, secure product supply.
Here are eight aspects of supply chain management that we have found ensure product quality and availability are never sacrificed.
1. Risk Management
The proper management of risk helps control future outcomes through the proactive identification of risk factors, the determination of a risk’s impact, and the development of risk mitigation and prevention strategies.
 For example, we have a dedicated Enterprise Risk Management (ERM) team at Neuland. This group of experts employs agility and foresight to ensure prompt and adequate responses to unforeseen risks. The team keeps a finger on the pulse of rapidly evolving demand patterns and consumer behaviors. They readily anticipate the need to reconfigure current strategies, processes, structure, personnel, or technology.
For example, we have a dedicated Enterprise Risk Management (ERM) team at Neuland. This group of experts employs agility and foresight to ensure prompt and adequate responses to unforeseen risks. The team keeps a finger on the pulse of rapidly evolving demand patterns and consumer behaviors. They readily anticipate the need to reconfigure current strategies, processes, structure, personnel, or technology.
Rather than wait until a problem occurs, we proactively review potential scenarios and develop strategic answers in case a scenario materializes. The ERM team ensures viable options are already in place when unforeseen events arise.
The agility of this group was particularly evident during the COVID-19 pandemic. Neuland was able to navigate the challenges with minimal impact on our operations. Our effective risk management has been a testament to our reliability as a business partner and has made us a preferred choice for customers attempting to navigate the unpredictable global environment.
2. Vendor Depth and Selection
A ready pool of qualified vendors is necessary to ensure that there are sufficient suppliers. If a supplier is unavailable or becomes unsuitable, an approved backup should already be in place. The security of the supply chain is jeopardized any time an essential supplier vacates its position in the chain or performs at a subpar level. Proper manufacturer selection can result in:
- Improved supply chain security.
- Better traceability from the starting material to the final drug product.
- Reduction in time from production to commercial launch.
- Reduced development costs.
Upstream processes significantly impact downstream processes. With raw material suppliers, for example, we use an active collaboration approach. We involve suppliers early in the development process, identify and strengthen areas of improvement prior to commercial supplies, and set clear expectations on specs, methods, governance and sustainability. This also includes multiple shipment tracking re-opening schedules, and closely monitoring outstanding receivables.
Pharma companies should always work with active pharmaceutical ingredient (API) contract manufacturers that are familiar with regulatory starting materials (RSMs), understand the chemistry, and have well-established sourcing procedures in place.
3. Logistics Improvements
As discussed above, logistics challenges have increased due to the growing complexity of drug compounds. Drug sponsors should be aware of the logistics dependencies their compound demands, and what potential impacts could occur as a result.For example, Neuland has secured new partnerships with GDP-certified logistics organizations. These new collaborations have expanded our ability to meet the needs of our customers without jeopardizing the quality standards.
4. Database Searches
When looking to enhance supplier management, a thorough and consistent review of high-quality certified databases, such as Directory of World Chemical Producers (IVQIA) and Row2Technologies, is essential. A deep search and a due diligence can unearth new sourcing options for specific APIs and their intermediates. Search results can be lackluster, however, if NCEs are involved and commercial RSM options are limited. When options are restricted, a research and development team may be called upon to develop a cost-efficient manufacturing process that can facilitate manufacturing through an external source.
5. Embracing Technology to Improve Operations & Supple Chain Visibility
Embracing industry advancements is crucial for any pharmaceutical company who wants to remain flexible, reduce costs and maximize supply chain visibility. Advancements in flow chemistry and enzymatic chemistry which improve yields and process efficiency are examples of technology impacting operations – and ultimately supply chains. At Neuland, for instance, we’ve found they can shorten processing times, increase production capacities, increase purities, and more.
At Neuland, for instance, we’ve found they can shorten processing times, increase production capacities, increase purities, and more.
Another aspect of technology – the digitization of our supply chains – data analytics, AI and automation are being leveraged to improve process efficiency and precision. Data-driven insights are helping to lower costs, improve forecasts and prioritize consumer health. Having real-time supply chain data is a must. It brings new levels of understanding to mixed-level details and material capacities, which in turn helps optimize production.
6. Inventory Management
Inventory management can be a trade-off, exchanging market service for servicing costs. Operationally, unavailability results in longer cycles, expiry-related issues, and regulatory requirements that reduce flexibility from inventory management. This is a double threat for generic drugs that straddle global markets, combining the regulated with the less regulated. Additionally, demand patterns have evolved with the pandemic, becoming more erratic. This has further accentuated the complexity of pharmaceutical supply chains. The risk of stock accumulation is also an important consideration. Some stocks should be increased at various localities given the risk of supply chain disruption from unforeseen events, while others should not.
7. Supply Chain Sustainability
Sustainable supply chains should remain a priority in your de-risking strategies. As an illustration, we have enhanced our supplier assessment matrix and added items that help lower supply chain risk. The assessment covers 80% of essential suppliers and 40% of all procurement. However, by 2025, we plan to cover 100% of suppliers in the sustainable supplier assessment plan. Our Supplier Sustainability Assessment Matrix includes the following assessments:
- Resilience: De-risked business
- Reliability: Raw material quality and delivery performance OTIF
- Inclusivity: Supplier diversification & digitization (equal opportunity mechanism)
- Environment and social: Qualified & certified vendors having ISO 9001/14001/45001 certification
 Due to our commitment to sustainability, we were awarded the prestigious Silver Sustainability Rating by Eco Vadis. Although we have made great strides, we continue our quest for all-inclusive, sustainable growth in the pharmaceutical industry.
Due to our commitment to sustainability, we were awarded the prestigious Silver Sustainability Rating by Eco Vadis. Although we have made great strides, we continue our quest for all-inclusive, sustainable growth in the pharmaceutical industry.
8. Proactive Readiness
We’ve all learned that supply chain issues can arise unexpectedly and should be addressed promptly. We’ve found it best to take a proactive approach based on risk analysis. For example, Neuland maintains full visibility throughout the supply chain, allowing us to anticipate and avoid possible pitfalls.Being proactive helped us avoid prospective supply chain interruptions at the height of the pandemic. By increasing the inventory of key precursors that were critical to the functionality of our suppliers, we were able to repeatedly circumvent potential shortages and mitigate costs.
Interested in learning more about how Neuland leverages strong supply chain management practices to keep your drug API project on track and secure? Let’s chat – contact us today!
Advancing a Cholesterol Lowering Drug at the Height of the Pandemic
Posted on April 18, 2023 by Saharsh Davuluri
How Neuland Expanded Esperion’s Bempedoic Acid Amid Pandemic Lockdowns and Supply Chain Interruptions
 Just as COVID was making itself known in February 2020, the FDA was approving Esperion Therapeutics’ new first-in-class medication for the treatment of adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease who require additional lowering of LDL-C.
Just as COVID was making itself known in February 2020, the FDA was approving Esperion Therapeutics’ new first-in-class medication for the treatment of adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease who require additional lowering of LDL-C.
The emerging coronavirus and the FDA approval would converge as Esperion planned on expanding Bempedoic Acid supply at Neuland in 2020, setting up a challenging manufacturing environment for this potentially ground-breaking drug.
Esperion Therapeutics’ NEXLETOL® & NEXLIZET®
Esperion Therapeutics began working with Neuland Labs prior to the pandemic to support expansion of Bempedoic Acid, the major active ingredient in NEXLETOL® and NEXLIZET®. In 2019, we entered into a technology transfer and supply agreement for the subsequent commercial API production of Esperion’s New Chemical Entity (NCE), bempedoic acid.
Overcoming a Challenging Pandemic Manufacturing Environment
The Esperion project encompassed technology transfer, pilot batch production, scale-up studies, process validation and production of commercial quantities of the Bempedoic acid API.
Neuland and Esperion worked together to put supporting processes in place. This ensured Esperion’s Process, Analytical, Engineering and Supply Chain teams possessed remote monitoring capabilities and could provide virtual guidance in real-time.
Developing and implementing this level of communication and access proved critical to every facet of the project, from technology transfer and production of pilot batches, to process validation (PPQ), global regulatory filings and – ultimately – commercial production. All documentation was reviewed and approved online by Esperion. The intercompany team reviewed the project twice weekly, with technical meetings focusing on scale-up of both the intermediate as well as bempedoic acid API.
 Managing Through an Operational Slowdown
Managing Through an Operational Slowdown
In terms of manpower management, lockdowns and strict safety protocols meant that our staff strength was, by necessity, reduced to 50% of pre-pandemic levels. To account for these manpower constraints, we prioritized the team working on Esperion’s bempedoic acid.
Facility and equipment readiness during the lockdown also posed additional challenges. Systems which were idled for extended periods needed to be prepared and brought back online.
Managing Difficult Supply Chains
COVID caused a slow-moving (and still – to some degree – ongoing) supply chain challenge. As the pandemic progressed through early- and mid-2020, two supply chain complications became all-too-common: uncertainty and interruptions.
While unsurprisingly, supply chain logistics did cause challenges in import/export, shipping of reference standards, impurity standards and samples, they were generally minimized.
We had already taken steps to ensure alternative supplier readiness in the event of supply chain delays or disruptions. In this particular case, however, the client’s vendors were already qualified. This meant that the project could rapidly shift to validation and commercialization work.
Outcome: Project Success
Our team was tasked with implementing the process outlined during technology transfer. The process and specifications were therefore in-line with the expectations set by Esperion. Consistent and regular remote collaborative work with the various in-house teams at Esperion allowed us to complete technology transfer, process validation and approval of Neuland in the global regulatory filings on-time.
We’re proud to say that the story does not end there. After successful scale-up, commercial production of the bempedoic acid was shifted from Neuland’s R&D Centre to one of the manufacturing units in Hyderabad, India for continued commercial production of global supply.
In late 2022, Esperion announced that their Cholesterol Lowering via Bempedoic acid, an ACL-Inhibiting Regimen (CLEAR) Outcomes trial met its primary endpoint, demonstrating statistically significant risk reduction in MACE-4 in patients treated with 180 mg/day NEXLETOL® compared to placebo.
Neuland is honoured to play a critical role with Esperion, providing prescribers innovative options to reduce LDL-cholesterol and overall potential cardiovascular risk for patients.
We continue investing in agile development and manufacturing capabilities to quickly respond to our customer’s needs. With the experience of working with Esperion, we have been able to successfully place processes to manage projects effectively minimising the need for on-site or in-person interactions.
While the pandemic may be behind us, we are seeing an increasing need for a CDMO to be self-reliant and self-driven to execute API expansion projects. In that context, working with Esperion has been a highly enriching experience for Neuland.
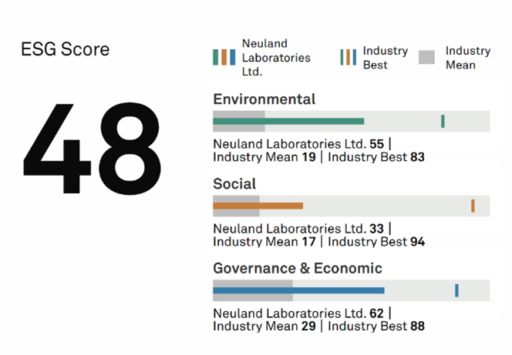
ESG in Pharma: Building Sustainable Businesses
Posted on April 1, 2023 by Saharsh Davuluri
 A study by research and sustainability data firm ESG Book has concluded that investments in companies with good ESG performance have generally yielded higher returns than the average within their broader market.
A study by research and sustainability data firm ESG Book has concluded that investments in companies with good ESG performance have generally yielded higher returns than the average within their broader market.
“The overwhelming weight of accumulated research finds that companies that pay attention to environmental, social and governance concerns do not experience a drag on value creation—in fact, quite the opposite,” noted a McKinsey report published in 2016.
Pharmaceutical companies are in a unique position when it comes to ESG. They work with chemicals – some of them dangerous if workers are exposed or they are disposed of improperly. They make medicines that can save lives, provided individuals can affordably access them. For this reason, pharmaceutical companies’ ESG profiles are increasingly being used to evaluate overall business sustainability.
A Short and Sweet Explanation of ESG
The “E” element in ESG references Environmental impact and sustainability. It considers how effectively and efficiently companies are using natural resources and how they are minimizing the impact of manufacturing and supply chain to reduce environmental consequences. Specifically, the “E” element may involve climate change, waste management, carbon emissions, air and water pollution, energy efficiency, natural resources management, sustainability practices, and water conservation. In the pharmaceutical industry, it includes the adoption of “green chemistry” practices.
 “S” is the Social element that focuses on how companies treat their workforces. This involves everything from diversity and inclusion to human rights, health and safety, security, and ethics.
“S” is the Social element that focuses on how companies treat their workforces. This involves everything from diversity and inclusion to human rights, health and safety, security, and ethics.
The “G” element in ESG refers to Governance, which involves decision- and policy making, as well as company structure – including the board of directors, managers, shareholders, and stakeholders. Some key indicators are how well the organization is run in terms of business ethics, board composition, reporting, transparency, strict adherence to local laws and environmental rules, risk and crisis management, tax strategy and anti-corruption, and integrity.
Benefits of a Robust ESG Program
While the benefits to the planet are self-evident, the benefits of an ESG program to corporate health and profitability are more nuanced.
Employment advantages. Increasingly, workers – particularly those of the younger generations – use ESG policies to determine which job offers they will accept. This can help limit employee turnover as well as recruiting, hiring, and training costs.
Investor attraction. Investors are becoming aware that higher ESG performance corresponds with higher rates of return, lower risk and better long-term corporate sustainability. A survey conducted last year by Gallup revealed that nearly half of investors – 48 percent – indicated they are very or somewhat interested in purchasing sustainable investing funds. The study also found that 70 percent of investors who are employed full or part time indicated they would definitely (13 percent) or probably (57 percent) include sustainable investing funds as elements of their employer-sponsored 401(k) if they were available.
Avoidance of fines. Misbehavior by manufacturing companies can lead to expensive fines and lawsuits. In the last decade, major pharmaceutical companies have paid out billions in fines for everything from false claims, bribery, off-label promotion, kickbacks, and Medicare fraud. Additionally, careless labor practices can result in expensive occupational injuries and accidents.
More productive employees. Responsible companies are taking employee health more seriously, from COVID-prevention strategies to onsite awareness programs about cardiovascular health, diabetes, stress and diet as well as occupational health-related training. Healthier employees lead to fewer sick days and lower turnover.
A reduction in operating costs. Asset management and predictive maintenance are strong elements of a good ESG policy, and done properly, they can lead to less downtime in manufacturing operations.
Corporate reputation burnishing. Pharmaceutical companies are in a unique position to help people access life-saving drugs at affordable costs. With high drug costs and anti-competitive behavior showing up regularly in the headlines, pharmaceutical companies that build a strong program which gets medications to the people who need them at affordable costs will stand out.
Transparency. At the core of a good ESG program is a boost in awareness and engagement for employees and stakeholders to become active parts of the change. By sharing the main environmental challenges, their objectives and the best practices from the field, companies can multiply the impact of their efforts.
At Neuland, we recognize that the raw materials we draw from the environment for our operations are shared resources, and that we have an obligation to minimize the impact of our operations on these resources and preserve them. For instance, we have implemented a water management strategy that focuses on reducing water consumption and increasing the percentage of recycled water in our operations.
As an organization, we ensure that support is extended to employees in performing their duty towards the environment through a dedicated budget and goal setting to measure the implementation of policies and ISO systems.
All of our sites have adopted a “Zero Wastewater Discharge” approach. During the rainy season, surface water runoff is collected and transferred to the CETP in accordance with the pollution control board’s statutory requirements. Effluent quality is evaluated daily, and third-party monitoring occurs monthly. Finally, we have put in place a strong program for material efficiency that seeks to attain optimal use of raw materials as well as practices that minimize the environmental impact of raw material use.
We have successfully achieved our 2022 environmental goals with:
- 2% reduction in direct greenhouse gas emissions
- 10% reduction in water withdrawal
- 34% recycling of waste
- Zero waste to land fill.
Our new environmental goals include becoming carbon neutral across our internal operations by 2030 and purchasing 100 percent renewable energy and influencing similar action across our supply chain. Our initiatives have been approved by the United Nations Global Compact (UNGC) and achieved a Silver rating in ECOVADIS sustainable supplier assessment.
Learn more about Neuland’s ESG initiatives and use of Green Chemistry.
How Neuland’s Process Engineering Lab Supports Robust Design and Minimizes Risk
Posted on February 6, 2023 by Saharsh Davuluri
 Drug developers know that processes which operate in a defined design space can be made more consistent, safer and less prone to deviation.
Drug developers know that processes which operate in a defined design space can be made more consistent, safer and less prone to deviation.
At Neuland, process development begins in the R&D laboratory with the application of rigorous scientific and engineering tools and methodologies, using Quality by Design (QbD) principles, risk management strategies, and Design of Experiments (DoE) to understand and control processes.
When applied to process development and scale-up, QbD focuses on five key issues:
· Critical Process Parameters (CPP)
· Critical Quality Attributes (CQA)
· Critical Material Attributes (CMA)
· Environment, Health & Safety (EHS)
· Project economics
The objective is to develop and scale a robust, reliable process and transfer it to the manufacturing facilities to ensure that quality product is delivered to our customers every time.
Putting QbD into practice
Over the past few years, we’ve expanded our capabilities and equipment with state-of-the-art instrumentation and automation to support QbD approaches with a dedicated Process Engineering (PE) Laboratory. Neuland’s PE Lab is staffed by highly qualified engineers and chemists who are trained in QbD and DoE.
The PE Lab utilizes two DoE software platforms:
- Minitab 20 Statistical software
- Design Expert statistical software from Stat-Ease Inc.
Based on experiments to identify the critical quality attributes (CQAs) of an active pharmaceutical ingredient (API) and the critical process parameters (CPPs), our scientists create a robust design space with flexibility built-in to maximize accuracy and reproducibility on scale-up and transfer to a manufacturing facility.
 Process monitoring and trend data are collected, analyzed, and used to develop a strategy for continuous process improvement in close collaboration with our customers.
Process monitoring and trend data are collected, analyzed, and used to develop a strategy for continuous process improvement in close collaboration with our customers.
The PE Lab also has provisions to assess the performance of processes in cylindrical reactors (using scale-down to mimic plant conditions). This can help avoid surprises when scaling up at the manufacturing plant due to potential differences when mixing in cylindrical reactors compared to round-bottomed flasks.
Process safety is critical
Robust process safety practices are essential for any process scale up. Our goal is to prevent the unintentional release of chemicals, energy, or other potentially dangerous materials during the course of chemical processes that can harm workers, equipment, a manufacturing facility, or the environment.
Engineers and scientists in the PE Lab design and conduct process safety studies to understand and assess the potential hazards in processes developed for our Custom Manufacturing Solutions (CMS), Generic Drug Substance (GDS), and Peptides business units.
The results of these studies can then guide the design and engineering of process controls and the development of a risk mitigation plan to reduce the chances for runaway reactions and ensure inherently safer processes at commercial scale.
Here are a sample of process safety practices and equipment commonly used in the PE lab.
Desk screening
Preliminary hazard evaluation/desk screening studies of the reactions are performed. This includes:
- Estimation of bond energies
- Estimation of oxygen balance, using a group contribution method
- Use of CHETAH software (ASTM international) to determine chemical thermodynamics
- Review of case studies from Bretherick’s Handbook of Reactive Chemical Hazards
- Performing a relevant literature search
Thermal screening
The PE Lab uses a Thermal Screening unit (TSu) to study the thermal stability of molecules at high temperatures. The TSu can assess the effects of elevated temperatures (process temperature + 100oC) on:
- Reaction mixtures
- Distillation residues
- Intermediates/final APIs
- Mother liquors (if recovered)
The TSu uses only approximately 0.5-5g of a sample. Screening studies evaluate the effects of the reaction initiation temperature on molecular stability and of pressure and heat release on chemical decomposition.
Reaction Calorimetry
 The reaction calorimeter in the PE Lab assesses the estimated energy released or absorbed during a chemical reaction. It measures:
The reaction calorimeter in the PE Lab assesses the estimated energy released or absorbed during a chemical reaction. It measures:
- Rate of energy liberation
- Enthalpy
- Specific heat capacity
- Adiabatic temperature rise
- Rate of gas generated
- Overall heat transfer coefficient (U) estimated from UA
This information is useful for understanding the severity of a chemical reaction and – based on the data – Neuland engineers and scientists can recommend control measures to be implemented at plant-scale to ensure inherently safer processes for producing commercial batches.
We’re a big proponent of data-driven approaches. The entire point of drug development is to maximize safety, accelerate development timelines and make the best possible decisions based on an appropriately designed experimental space. QbD fills this vital role of improving and accelerating drug development and commercialization projects.
Have questions about QbD or process engineering? Contact us to see how we can help!
Putting Customers First: Customer Centricity in Pharma API Manufacturing
Posted on January 1, 2023 by Saharsh Davuluri
 Customer centricity is central to every organization and an important strategic priority for B2B companies.
Customer centricity is central to every organization and an important strategic priority for B2B companies.
While there are many definitions of customer centricity, the ultimate objective of a customer-centric organization is to deliver the desired value customers believe they will receive from a product or service.
This customer experience, in turn, creates value for your organization and translates into repeat business, loyalty and advocacy. Among B2B organizations, customer centricity means meeting customer requirements at an agreeable price while ensuring compliance with regulations and ethical standards.
The strategic concept, developed by renowned management consultant and educator Peter Drucker, prioritizes the needs of the customer.
Drucker birthed the idea in 1954, stating:
“It is the customer who determines what a business is, what it produces, and whether it will prosper.”
Customer centricity hinges on an organization’s ability to anticipate the needs and desires of the customer. Once those needs have been deciphered, a customer-centric company ensures the requirements are met to the customer’s expectations.
 The success of the selling organization and that of the B2B customer are strongly interrelated. The B2B customer expects a positive experience, and the selling company desires the organic growth that results from true customer satisfaction. The customer-centric concept promotes customer interaction and respect—two ideals critical to improving the overall customer experience.
The success of the selling organization and that of the B2B customer are strongly interrelated. The B2B customer expects a positive experience, and the selling company desires the organic growth that results from true customer satisfaction. The customer-centric concept promotes customer interaction and respect—two ideals critical to improving the overall customer experience.
Customer centricity involves transformative changes that redirect the focus on customer retention instead of just acquisition. We believe that Jonathan Hughes, David Chapnick, Isaac Block, and Saptak Ray perfectly captured the scope of change necessary to become a customer-centric company:
- Understanding your customers and their expectations
Customer centricity begins with understanding the unmet needs of your customers, what is that you can provide to support the end result, and what is the desired result they would like to see addressed through your partnership. This is often easier said than done but being committed to meeting expectations drives differentiation and makes customers feel valued. - Deliver seamlessly, keeping customer centricity at the heart of everything
Delivering to customer expectations – or surpassing them – is imperative. The entire process of understanding customers must be seamless and transparent to them. Customers like to be involved, aware and updated, so you must give them access to tools which provide the status of projects while also enabling continuous feedback. - Go above and beyond
It is important to differentiate your company from the competition by adding value that exceeds expectations. We look beyond each customer’s stated need, and invest in delighting them. In the pharma industry, for example, this differentiation occurs when the customer-centric organization proactively anticipates expectations and successfully resolves any issues or impediments before they escalate – or come to the attention of the customer. - Seek feedback regularly
How do you identify, adapt to, and manage the changing needs of customers? The most effective way is to seek feedback on an ongoing basis. Feedback allows you to track and measure areas that meet customers expectations as well as those areas needing improvement. Customer feedback surveys also connect you with your customers, and demonstrate that their needs are a central focus of your organization.
Feedback builds trust and brand loyalty, helping strengthen customer retention. This is even more valuable in cases where the feedback has been negative, as it gives you insight into what is needed to address their needs or concerns.
At Neuland, customer centricity is a strategic priority, and we recognize that a customer may not always share unsolicited feedback directly. However, by seeking feedback through surveys and consistent communication, we can gather and analyze behavioral data, then use the information to make strategic operational improvements that deliver desired outcomes.
We find excellent service throughout the entire customer experience, coupled with transparency, leads to greater customer involvement, awareness, and ultimately satisfaction.
We choose not only to meet client expectations but to exceed them through proactive anticipation. We look beyond the expressed need to ensure that we resolve potential failures before they occur.
What’s the value of a customer-centric pharmaceutical API manufacturer?
It means meeting customer requirements at an agreeable price without sacrificing regulatory compliance or ethical standards. Ultimately, the value of customer centricity aligns with our purpose at Neuland: to deliver product and service value to our customers in a way that fosters loyalty, advocacy, and strong ongoing partnerships.
Discuss your next project with us today.
Planning for An Unpredictable 2023: Effective Pharma Risk Management Strategies
Posted on December 2, 2022 by Saharsh Davuluri
Congratulations! The last three years have been a Ph.D. in Supply Chain Disaster Management for Supply Chain teams!

It’s undeniable that the last few years have highlighted a variety of risk factors which can dramatically impact the pharmaceutical industry.
In this ever-evolving environment, effective risk management processes are essential to keep supply chains running smoothly. They can also help achieve other strategic and operational goals.
 Recognizing this, over the last year Neuland Labs has undertaken an Enterprise Risk Management (ERM) enhancement program. The objective was to ensure we had a comprehensive system for identifying risks and monitoring mitigation plans in place.
Recognizing this, over the last year Neuland Labs has undertaken an Enterprise Risk Management (ERM) enhancement program. The objective was to ensure we had a comprehensive system for identifying risks and monitoring mitigation plans in place.
Neuland’s board approved a new ERM policy, quantitatively rating a variety of risks based on severity and likelihood, while adding an additional layer of analysis and review. Risk identification workshops were held across our organization, which led to an expansion of the potential threats and challenges assessed by the program.
Some of the key risks addressed by the program are listed below, along with steps the company is taking to mitigate them.
- Supply Chain Risk
As everyone now understands, supply chain risks are a critical issue across the industry. One particular area of concern – not just in India, but also in the U.S., EU and elsewhere – is the level of dependence on China for imports, especially in API manufacturing.
Since any production disruption can seriously hamper our client base, controlling supply chain risks is one of our top priorities. As a result, our aim has been to continuously diversify and reduce our distance from suppliers, working in close coordination with our sales and R&D teams for raw material planning and availability.
As a result of these efforts, we’ve already reduced our global dependence for raw materials to 13%. We are working to reduce this dependency even further (to less than 10% by FY 2022–23) through ongoing geographic de-risking efforts. In addition, we’re working to create two or more active and dependable sources for Key Starting Materials (KSMs) and critical intermediaries, manufacturing them domestically or in-house when possible.
- Intellectual Capital Risk
Human capital is key to our R&D and operations, ensuring business sustainability and growth. As you know, it is particularly important in today’s labor environment.
We’ve enacted HR policies designed to ensure sufficient highly qualified staff. Employees who show promise for greater work responsibilities are internally promoted and provided with training and skill development programs. In-house talent is also considered for middle and senior managerial roles, though we’ll recruit from outside sources if necessary.
We work to maintain and increase employee satisfaction with an open-door policy, healthy work environment, job rotation and other initiatives for retaining talent. In addition, we’re working to attract talent from abroad, multinationals, post-doctoral programs, and the chemical engineering cadre.
- Regulatory Risk
In recent years, we’ve seen an increased dependence on regulated markets for revenue, making non-compliance a potential threat to operations. In response, we emphasize health & safety – and boast multiple global certifications (some of which we exceed).These initiatives include a growing investment in automation of quality management systems and monitoring of metrics through QA Dashboards to enhance efficiency and accuracy. High standards of regulatory compliance are promoted across all operating units, and any customer issues are handled through a structure designed to ensure that any incidents are not repeated.
 We have established a stringent internal monitoring and review mechanism. All exceptions are directly communicated to the CEO and/or Quality Head. We have also initiated third-party quality audits.
We have established a stringent internal monitoring and review mechanism. All exceptions are directly communicated to the CEO and/or Quality Head. We have also initiated third-party quality audits.
- Product Scaling Risk
Product portfolio expansion is imperative. We work to ensure seamless scalability of products from R&D through to our manufacturing units. Any product scaling issue could potentially cost the company a new or existing customer. Detailed checklists are used, confirming that every aspect of the process is done right the first time to ensure unified product transfer.
- Geopolitical Risk
Trade conflict between any two countries has a direct or indirect bearing on economies globally. Our wide geographical footprint ensures our operations are not heavily impacted by disruptions in any single economy. We have also diversified our supplier base to ensure uninterrupted supply in the event of unforeseen circumstances.
- Competition Risk
Intense competition and mature product portfolios can have a negative impact. Our mitigation strategy includes dynamic life-cycle management, as well as a focus on both maintaining our current customer base and nurturing new partnerships.We are exploring innovative areas to serve new clients and we are targeting larger volumes for specific molecules.
- Emergency & Unforeseen Events
Agile practices and operating models enable organizations to solve specific problems quickly and efficiently. During the COVID-19 pandemic, we’ve proven our ability to face challenges with minimal impacts on operations.The agility and foresight of our ERM team enables us to initiate a prompt and adequate response to any unforeseen risk. The team closely monitors rapidly evolving consumer behaviors and demand patterns, anticipating potential reconfiguring of strategies, structure, processes, people, and/or technology.
Neuland actively works with alternate raw material suppliers, tracking re-opening schedules, clubbing shipments, monitoring outstanding receivables, truck transit and arrival closely and more to ensure efficient supply chain management. In some cases, it is important to consider stock accumulation risks and whether stocks should be built up for various geographies given the risks of supply chain recovery due to an unforeseen event.
Risk management aims to control future outcomes by proactively identifying the possibility of a risk occurring, determining what its potential impact could be, and developing strategies to mitigate or avoid those risks. Effective risk management gives Neuland the resilience as a preferred and reliable business partner to help our customers succeed in an unpredictable environment
End-to-End Focus on Quality in API Manufacturing
Posted on October 31, 2022 by Saharsh Davuluri
 The past few years have seen major changes to the ways in which drug companies and their regulators go about ensuring the safety and efficacy of drugs. The response to COVID-19 accelerated trends that were already defining an era of significant change.
The past few years have seen major changes to the ways in which drug companies and their regulators go about ensuring the safety and efficacy of drugs. The response to COVID-19 accelerated trends that were already defining an era of significant change.
As scientific knowledge continues to advance, both in the fields of human biology and complex synthetic chemistry, most companies are either in the process of transitioning to digital data collection and retrieval or have already completed it. Manufacturing and Quality processes are also being streamlined and automated wherever possible.
Modernization is affecting everything from analytical instrumentation and process monitoring to risk management practices.
This work is enabling new levels of end-to-end visibility, helping to advance the goal of quality-focused cultures throughout the drug manufacturing industry. This is particularly important as globalized supply chains continue to expand, despite ongoing trade and supply chain uncertainties.
All of these factors have contributed to the increasing complexity of compliance as well as the importance of compliance. As a result, regulatory and quality excellence matters more than ever before.
The Growing Importance of Process Validation
Process validation has evolved significantly since it was first proposed by FDA officials as a one-time event to improve pharmaceutical quality in the 1970s. No longer merely a one-time “box-checking” exercise, it’s now an ongoing legal requirement in the drug industry, designed to build quality into every step of the process.
Good manufacturing practices (GMPs) for finished pharmaceuticals require drugmakers to determine that manufacturing processes can consistently meet finished product quality requirements, including those characteristics impacting the quality, purity and potency of a compound. This means your CMO must understand:
- when variation occurs in a process
- what the source of the variation is
- how the variation impacts both processes and products
- how variation can be controlled.
As the FDA makes clear in its Guideline on Process Validation: General Principles and Practices, this typically involves teams with “expertise from a variety of disciplines (e.g., process engineering, industrial pharmacy, analytical chemistry, microbiology, statistics, manufacturing, and quality assurance).”
Three Key Process Validation Stages
Process validation is broken down into stages, as follows:
- Stage 1: Process Design
In this first stage, the manufacturing process is designed to ensure a consistent ability to meet target quality attributes. The key to sound process design is thorough documentation, which becomes essential in subsequent stages. Process design often includes Design of Experiment (DoE) studies, risk analysis tools and the results of verification runs at lab or pilot scale. This collective information can help predict performance of commercial scale processes. - Stage 2: Process Qualification
Process qualification refers to the qualification of facility, equipment, and utilities, as well as the manufacturing processes themselves. Once the facilities and equipment have been individually qualified, the process performance qualification (PPQ) can occur. Process qualification assesses the data gathered from all relevant studies, including experiments, lab-, pilot- and commercial batches. Successful qualification demonstrates that commercial manufacturing processes will perform as expected. - Stage 3: Continued Process Verification
Stage 3 relates to the ongoing activities that occur, reflecting the ‘lifecycle process validation’ approach in use today rather than the one-time approach common years ago. The objective of continued process verification is to ensure the process remains validated, that it is still in a “state of control.” To confirm this, drug and API manufacturers need systems in place to detect nonconformities in processes. One outcome of this stage is often process improvement or optimization strategies, though these are often subject to additional regulatory approval or further process validation.

Neuland’s Commitment to Quality Assurance and Control
To keep pace with both technological advances and evolving regulations, Neuland complies with stringent international standards. In 2022, we continued to invest heavily in quality. Policy implementation is prompt and at times ahead of legislation thanks to focused monitoring of possible trends in regulations and standards.
Key initiatives of fiscal year 2022 have included:
- The implementation of Laboratory Information Management System (LIMS) software across three sites, with full implementation to be completed by FY 2022–23
- Installation of a paperless document management system to increase accuracy and quality assurance
- First Time Right through robust Technology Transfers, a structured protocol checklist to reduce product product/process failures during scale-up
- Implementation of Project Management systems to enhance customer satisfaction via an automated process managing communications with customers, investigations, root cause analyses, and corrective & preventive actions (CAPA)
- Scalability of QA, QC teams in order to meet CMS customer needs
- Enhancement of the quality of investigations to ensure promptness in investigations and root cause corrections. Regular monitoring of process robustness, investigation status, report documentation, effectiveness of scientific rationale, and CAPA
- Expansion of document archives per guidelines, enabling all GMP documents and development products lifecycle reports to be stored for a minimum of six years
Want to explore Neuland’s commitment to a quality culture? Learn about:
Ensuring Rock-Solid IT Infrastructure in Contract Manufacturing
Posted on September 30, 2022 by Saharsh Davuluri
 Digital transformation went from a popular corporate buzz phrase to an essential survival tool with the outbreak of the COVID-19 pandemic. Industries ranging from banking and restaurants to travel and manufacturing had to re-invent their business models literally overnight.
Digital transformation went from a popular corporate buzz phrase to an essential survival tool with the outbreak of the COVID-19 pandemic. Industries ranging from banking and restaurants to travel and manufacturing had to re-invent their business models literally overnight.
Although the acceleration of digital adoption changed many industries for good, few saw as significant an evolution as the bio/pharmaceutical industry.
As a result, IT has not only gained center stage; it has become a significant business enabler and differentiator. The early needs of social distancing, remote work and even (for a time) off-site regulatory inspections have given way to a new era of collaboration, diversity and flexible production — all of which is occurring at a faster pace than ever before.
Although these changes continue to open exciting new doors for the industry — including more advanced applications for artificial intelligence (AI), analytics tools, long-distance training and the promise of precision medicine — they’ve also brought new needs and challenges, both for pharma companies and their clients.
Going forward, contract manufacturers and other pharma stakeholders will need to satisfy two critical demands when it comes to IT:
- Greater visibility throughout their supply chain operations.
- Improving operations by making them more adaptive and responsive.
Here’s a quick overview of key best practices that can help make these goals a reality, enabling contract manufacturers to better serve their customers while ensuring their peace of mind.
Establish IT Priorities
Before embarking on major IT investments, it’s important to have a clear vision of the capabilities and safeguards you will need to ensure smooth operations, data security and compliance. This was the process we pursued at Neuland, setting the following goals prior to our most recent round of upgrades:
- Full commitment to hybrid multi-cloud adoption
- SAP BPR, upgrades, and customer relationship management (CRM) systems
- Establishment of a data-driven digital enterprise with the adoption of AI and Big Data capabilities
- Digitization and intelligent automation initiatives to drive business growth
- Strengthen the organization’s cyber security posture by:
- Advancing XDR (enhanced detection and response) implementation
- SIEM and NSX (VMware’s Network Virtualization and Security Platform) implementations
- Adoption of a zero-trust framework
- Reinforce privacy to protect customers, clients, partners, and employees
- Build resilient IT operations with robust business continuity plans
- Enable remote quality audits with wearables and technology
IT Infrastructure
Significant investments in IT are imperative for any pharma company that wants to compete in today’s rapidly evolving marketplace. A robust IT framework enables effective and seamless workflows – not only at individual sites, but offices, remote workplaces and operations worldwide. IT modernization also delivers benefits by making pharma organizations more cohesive and closely connected.
The pandemic has also forced a rethink of technology practices. One method of reducing disease transmission and limiting sterility issues was mandating the use of facial recognition, along with a temperature control system. After determining that this was the safest and fastest method of security, such systems were implemented across all Neuland locations.
Virtual desktop infrastructure (VDI) was also established for up to 90% of the organization to improve hybrid working and security. This enabled critical data to be stored safely in one central location while also enabling users to have convenient remote access.
Enterprise Application
Identifying a “single source of truth” (SSOT) can significantly improve management of master data. It’s the core process we use at Neuland to manage, centralize, and organize master data according to the business rules of the sales, marketing and the operational strategies of our company.
Better data quality delivers significant benefits in the form of improved business processes and greater efficiency, coupled with the elimination of manual processes — and the opportunities for human error that accompany them. All of these initiatives can reduce the workload on your team, which is a  significant benefit as qualified talent becomes increasingly more difficult to find and retain. The adoption of robotic process automation (RPA) is also revolutionizing business operations by increasing productivity, enhancing accuracy and optimizing the use of resources in addition to the automation of the human process, response, and triggers.
significant benefit as qualified talent becomes increasingly more difficult to find and retain. The adoption of robotic process automation (RPA) is also revolutionizing business operations by increasing productivity, enhancing accuracy and optimizing the use of resources in addition to the automation of the human process, response, and triggers.
At Neuland we’re also working on further improving resource management – including new strategies for planning, scheduling, and allocating resources to the right project at the right time to maximize customer profitability. The company recently undertook IFC automation to improve its governance process and implemented contract workforce management for all locations to efficiently manage the attendance and payroll of contractors.
Data Security
Data protection and confidentiality are critical to any organization that works with innovator companies, especially in the pharmaceutical space. Regular vulnerability assessment and penetration testing (VAPT) should be conducted for any business unit operating critical devices. This ensures the resilience of IT infrastructure while enabling identification of possible routes attackers could use to break into the company network. In addition, security information and event management (SIEM) provides advanced threat detection so they can be addressed.
Other Best Practices
As remote collaboration, big data, and other information-rich needs continue to expand, it’s difficult to imagine how any pharma company can have too much bandwidth. We recently doubled ours across all global locations. Other effective initiatives include the implementation of network monitoring tools, centralized cloud-based WiFi infrastructure, and using applications such as Barracuda to provide email, spam and phishing filters.
3 Key Elements of a Successful Hydrogenation Scale-Up
Posted on August 30, 2022 by Saharsh Davuluri
 Pharmaceutical process chemistry has come a long way since wholesale merchants began the commercial marketing of drugs in the 19th century. In fact, process chemistry has driven many of the key milestones in drug development over the last 100+ years.
Pharmaceutical process chemistry has come a long way since wholesale merchants began the commercial marketing of drugs in the 19th century. In fact, process chemistry has driven many of the key milestones in drug development over the last 100+ years.
Hydrogenation is an excellent example of a proven chemistry technique that is vital to modern drug discovery and development.
Why?
Chiral chemistry. Hydrogenation drives the field of chiral API synthesis.
Hydrogenation for API Synthesis
Hydrogenation refers to a chemical process in which molecular hydrogen (H2) is reacted with another compound, typically in the presence of a catalyst such as palladium or nickel. Catalytic asymmetric hydrogenation gives rise to a diverse array of chiral molecules useful for producing APIs.
The process involves the reduction of unsaturated compounds, often alkenes, to introduce one or two chiral centers. In turn, chirality plays a critical role in shaping a drug’s pharmacology by refining its target selectivity.
More than half of drugs on the market contain one or more chiral centers to ensure better binding affinity and stereoselectivity. However, achieving ideal compound chirality can be challenging.
Hydrogenation is a practical and convenient technique that is well-established in pharmaceutical process development. Its popularity and broad use are attributable to several key factors, including:
- Versatility
Hydrogenation is capable of an impressive range of chemical transformations, one of the principal reasons for its use. As mentioned above, its role in generating chiral molecules has been vital to API synthesis. Hydrogenation processes can involve heterogeneous or homogeneous catalysis depending on the level of selectivity needed. Catalytic hydrogenation can be fine-tuned for efficiency by modifying a variety of factors, including reaction conditions, catalyst selection, and the use of flexible equipment and methodologies. - Efficiency
The catalytic hydrogenation of pro-chiral compounds shows constant high conversions and remarkable enantioselectivity. It is an economical, high-yield process that avoids the need for super high temperatures and does not generate large amounts of waste. These traits are why interest in hydrogenation processes – with an eye toward scalability – has become so prevalent among pharma companies. - Sustainability
Green criteria for catalytic hydrogenation include using a clean and abundant resource (H2), recovery and recycling of catalysts or unreacted H2, sufficiently low catalyst loading, minimalized use of organic solvents, and closed reaction systems to prevent harmful emissions to the environment. These are practical conditions that can easily be achieved via optimally designed hydrogenation processes utilizing suitable reaction parameters. - Simplicity
Hydrogenation isn’t rocket science (though it does require expertise). For example, hydrogenation processes often use simple batch multipurpose reactor technology. The unsaturated substrate, hydrogen, and the catalyst are completely blended inside a high-performance hydrogenation reactor with a well-designed agitation system. Filtration of reaction mass then separates the solid catalyst from the suspension of the hydrogenated substance.
3-Step Guide to Scaling Up Hydrogenation Processes
There’s no denying the appeal of hydrogenation from both an economical and environmental perspective, but – and ultimately this is one of the most important questions in drug commercialization – is it scalable? There are a number of scales between bench and multi-ton bulk manufacturing. Translating processes across those scales is rarely linear – or straightforward.
Hydrogenation is quite scalable, but success hinges on 3 things:
- A 3-Step Mass Transfer
Hydrogen treatment typically occurs within a three-phase reactor. It involves first mass transfer from gas phase to liquid phase, then liquid to solid phase, and finally transfer into the porous catalyst. Therefore, an optimized mass transport system is necessary to prevent variation in reactor performance.
- Hydrogen Delivery
A widely popular hydrogen feed system is the EKATO hydrogenation reactor, which uses hollow-shaft gassing agitator technology. The hydrogen is fed from the bottom of the hydrogenation reactor for dispersal into very fine gas bubbles. The best hydrogen feed system should allow for varying hydrogen delivery rates based on the required pressure build-up and specific point of process. Recycling unreacted H2 is also vital for economic reasons. - Thermal Control
Hydrogenation demands heat. However, the reaction becomes exothermic once the process gains momentum, resulting in temperature runaway. It’s important to maintain a tight rein on thermal and flow parameters which can impact reaction performance. Since massive amounts of heat must be dissipated (exothermic flux removal), performing thorough heat transfer calculations can minimize issues with heat exchange.
Command of these 3 essential concepts will streamline the step up to large-scale production from laboratory/pilot plant hydrogenation. In addition, it will prevent surprises once hydrogenation in the plant commences.
Contact us today and learn how Neuland can help advance your chiral chemistry project.
Curious about Hydrogenation at Neuland?
Neuland’s investment in state-of-the-art hydrogenation capabilities has delivered key successes to our clients – from enhanced product development capabilities and improved operational efficiency to expedited time-to-market and more flexible manufacturing arrangements.
Our hydrogenation capabilities span every step of the process and include the following:
- Development of hydrogenation processes.
Our expert hydrogenation team establishes optimized laboratory reactor conditions to facilitate meaningful process characterization before full-scale production. They apply their knowledge and appropriate tools, such as Differential Scanning Calorimetry (DSC) and Reaction calorimeter RC1, to conduct a thorough safety evaluation.
- Process scale-up.
We’ve developed a comprehensive toolbox of proven methodologies that incorporate various stereoselective transformations. Our process development and engineering work ensures successful, large-scale batch processes.
- Customer specifications.
The beauty of hydrogenation lies in its versatility and accommodation of a changing product spectrum. We use flexible technology and equipment to develop customer-specific hydrogenation processes that meet various specific needs.
- High-performance equipment.
State-of-the-art, industrial-scale equipment includes a high-performance hydrogenation reactor and batch hollow-shaft gassing agitator. These tools enable us to fine-tune highly specific operating ranges and efficiencies, whether in the lab or at the plant level.
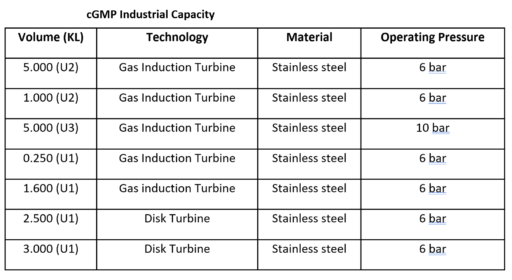
- cGMP compliant capacity.
Our industrial-scale, cGMP compliant hydrogenation capacities are as follows:
cGMP Industrial Capacity
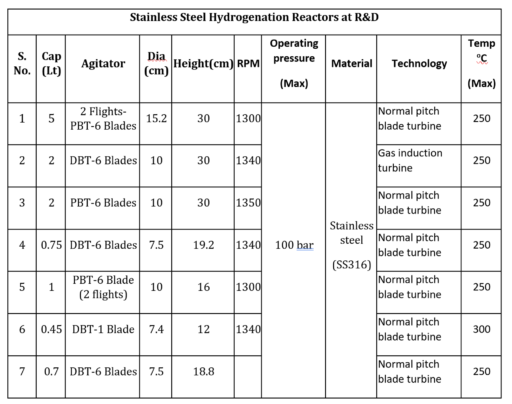
Neuland also offers substantial hydrogenation capabilities for R&D projects, allowing us to scale your process as needed from the bench through commercial production:
R&D Capacity
Pharma Industry Zeroes in on Drug Manufacturing Sustainability
Posted on July 31, 2022 by Saharsh Davuluri
 Sustainability has become a key trend in drug manufacturing. As we’ve previously discussed (here and here), this is being driven by environmental/good corporate stewardship efforts as well as various cost considerations. From greening supply chains to reductions in carbon emissions, pharmaceutical companies are increasingly taking steps to incorporate the principles of green chemistry into every aspect of their business.
Sustainability has become a key trend in drug manufacturing. As we’ve previously discussed (here and here), this is being driven by environmental/good corporate stewardship efforts as well as various cost considerations. From greening supply chains to reductions in carbon emissions, pharmaceutical companies are increasingly taking steps to incorporate the principles of green chemistry into every aspect of their business.
With organizational spotlights focused on sustainable practices, Environment, Health, and Safety (EHS) departments are receiving a great deal of attention.
The Challenging Role of an EHS Department
Although ecological responsibility and sustainability are popular buzzwords today, EHS teams don’t have an easy job. Much of pharma’s success, in fact, rests on the EHS department. From a ‘green business’ perspective, the department is responsible for developing and implementing policies that encourage sustainable practices throughout an organization. They also monitor the progress of those efforts.
From a ‘green chemistry’ perspective, they work closely with process and product chemists to ensure the safety of manufacturing processes. Without a competent team, the safety of the workplace, the employees, and the environment is at risk.
Effective EHS policies keep operations running smoothly toward the goal of sustainability, ultimately creating a healthy balance between risk mitigation and adherence to sustainable green principles.
EHS and Continuous Improvement
Even as EHS teams achieve successes and make strides in supporting sustainability, they remain aware that their work isn’t once-and done. Rather, it is an ongoing process focused on continuous improvement. Key functions of EHS team members include evaluating processes and practices, focusing on employee education through green training and setting measurable goals for green actions.
EHS Focus on Employee Health
To prioritize occupational health, an EHS team should offer training that covers personal hygiene, health monitoring, first aid knowledge, and adherence to COVID protocols. These health-related issues recognize the importance of human workers and are indispensable to the protection of company manpower. Many pharmaceutical companies are still rebounding from the detrimental effects of COVID-19 on the workforce. Protective measures that preserve employee health are crucial.
Additional EHS training that focuses on other areas of concern – such as chemical safety and emergency procedure – are also vital. The success of the safety-based employee education is often evidenced by a reduction in the number of negative incidents and failed audits that occur.
 Teams can monitor and facilitate company sustainability through:
Teams can monitor and facilitate company sustainability through:
- Routine site inspections. The goal is to identify safety concerns and rectify them quickly. The EHS department should offer one-on-one guidance when necessary, giving staff members as much assistance as they need to master safer techniques.
- Facility design reviews. As renovations are needed and layouts are planned, EHS experts recommend changes that adhere to safe practices supporting both employee and ecological health.
- Emergency drills. Drills can be used to expose employees to mock emergencies and help them prepare for the unexpected.
- Incident investigations. Once an incident occurs, teams should be prepared to lead an investigation to determine the root cause. Additionally, they must ensure the implementation of corrective and preventive actions to avoid a recurrence.
Drug Manufacturing Sustainability Practices
The sustainability practices EHS teams employ are largely directed towards the reduction of waste output. Below are additional practices that EHS teams use to promote sustainability:
- Reduction in solvent usage. EHS experts are identifying less-harmful alternatives to traditional solvents.
- Recycling of materials. By reusing materials, companies can reduce their environmental impact.
- Reduction in the use of natural resources. EHS departments advocate the conservation of natural resources.
Despite the challenges associated with greener API manufacturing, some drug API manufacturers – including Neuland Labs – are leveraging better synthetic route design and sourcing alternate chemicals, reagents or precursors. The results can include less effluent and pollution, higher yields, shorter processing times, the use and storage of lower volumes of volatile chemicals, and the ability to downsize manufacturing infrastructure.
EHS, Sustainability & Neuland Labs
All of Neuland’s manufacturing units have adopted policies that protect the environment – including climate change and energy policies to reduce the company’s carbon footprint and Zero Waste Discharge policies to help minimize waste to landfills.
One key aspect of sustainable chemistry focuses on minimizing waste disposal via incineration. Working towards a goal of 0% incineration of waste, we send our spent solvent and other material waste to cement manufacturers, who use co-processing technology to convert this waste to an auxiliary fuel.
We achieved a recovery rate of 80-85% in FY 2021 using sophisticated solvent recovery systems. A wastewater treatment plant with higher capacity also came online, enabling the efficient management of additional load on the effluent system due to operational surges.
Some of the additional changes we’ve implemented are:
- ISO 14001:2015 and ISO 45001:2018 certifications for all manufacturing units.
- Recycling of treated water. Neuland’s recycling efforts employ good water accounting to conserve water and reduce waste.
- Control of emissions. Scrubbers are used to improve air quality – reducing emissions and ensuring that they stay well below restricted levels.
- Management of hazardous waste. The collection, storage, transport, and disposal of the waste are governed by strict guidelines that adhere to regulatory standards of Hazardous Waste Management Rules, 2016.
- Water runoff storage.
- Specialized liquid chemical storage.
By assuming global good stewardship practices and incorporating EHS-focused actions, the pharmaceutical industry is well-positioned to optimize opportunities to protect the world’s citizens and ecological systems.
Related Blog Content
EHS Stewardship: Green Chemistry, Healthy People, Healthy Workplace
Green Generic Drug Substances: Increased Upfront Costs Offset by Long-Term Profitability
API Production: Building a Process Safety Culture
Resources
Learn more about Neuland’s Environmental, Health and Safety (EHS) Team.
Learn about Corporate Social Responsibility at Neuland Labs.