 Selecting the right pharmaceutical contract manufacturing partner for Phase 3 clinical and commercial supply is challenging. There are several critical qualifiers you need to consider:
Selecting the right pharmaceutical contract manufacturing partner for Phase 3 clinical and commercial supply is challenging. There are several critical qualifiers you need to consider:
- Do they have the expertise needed to resolve the limitations of the existing process chemistry and ensure a smooth scale-up?
- Are they experienced in handling large scale sensitive reagents and reactions?
- Do they have route scouting and development expertise?
- What is their regulatory track record? Can they get you to your target clinical or commercial stage?
- Does the contract pharma partner help your company solve supply chain challenges and reduce risks?
- Can the partner help accelerate speed-to-market?
Here’s how Neuland and Karuna Therapeutics partnered to meet the company’s clinical and commercial supply objectives.
Karuna Therapeutics is a biopharmaceutical company developing drugs for people living with psychiatric and neurological conditions. When Karuna and Neuland started discussions in early 2019, Karuna had an abbreviated timeline to scale up an NCE API, produce clinical trial quantities, and begin commercial manufacturing to support a combination CNS drug.
Tackling Challenges to Meet Phase 3 Clinical Trial Deadlines
Karuna and Neuland started working on the project in mid-2019. The scope of work included:
- Balancing development of the chemistry and scalable processes along with meeting material requirement for clinical trials
- Preparation of impurities, impurity identification and profiling, fixing of specification of raw material and intermediates
- Identification and vendor qualification for raw material
- Process safety studies
- Impurity spike and purge studies
- Scale-up manufacturing to meet requirements for Phase 3 clinical trials
- Design of Experiments
- Characterization and consistency in solid state properties like polymorphism, particle size distribution, flowability of material and morphology of the crystals
- Process Validation (PPQ) including Analytical method validation
- NDA Registration Batches
- Commercial manufacturing
- Performing stability studies
- Providing CMC documentation and regulatory filing support.
Tech Transfer: Translating Process Chemistry
It’s widely known in the industry that technology transfer can be a source of complex problems. CROs and CDMOs who focus earlier in the drug development cycle (preclinical through Phase II, for example) tend to be less familiar with process scaling issues that may arise at late-stage clinical and commercial scales, such as large scale hazardous chemistry. This is something that often is not taken into consideration during early R&D or process development. Raw material sources specified in early process design (discussed below) can be another potential pain point as procurement needs shift to bulk quantities.
Managing Supply Chain Risks: Backward Integration Via In-House Synthesis
Karuna – like other forward-thinking pharma companies – saw the need to have robust global Regulatory Starting Material (RSM) supply chains. Cost, delays, shutdowns, backed up ports, insufficient trucking capacity – the industry was surrounded by logistics risks and Karuna needed to ensure supply security.
Due to unforeseen challenges with the original RSM, Karuna and Neuland decided it would be advantageous for the client if Neuland developed capacity for RSM. By doing so, Neuland backward integrated production of the RSM, allowing for high quality and cost-effective manufacturing, and also established three domestic secure sources for the RSM’s starting material.
Handling Facility Shutdowns and Global Logistics
Shutdowns due to COVID outbreak were a particularly worrisome supply chain issue. When China closed, and India moved to follow suit, Karuna asked Neuland to ship available material to secure it in a GMP warehouse in the U.S. for its ongoing clinical trials.
At the time, global logistics were in a state of chaos, but Neuland’s expert logistics team ensured sufficient material was pre-positioned in the U.S. to meet the Company’s immediate clinical needs.
Complex, Hazardous Chemistry at Scale While Improving Yield and Speed
From a process design standpoint, handling large volumes of sodium cyanide poses risks. The route development team at Neuland was able to eliminate multiple hazardous reagents and large-scale reaction conditions while increasing yield and reducing cost.
The original process design produced low yields at each of its four stages. A cross-functional team at Neuland evaluated this 4-stage process, identified potential gaps, and created greener, more efficient and cost-effective processes.
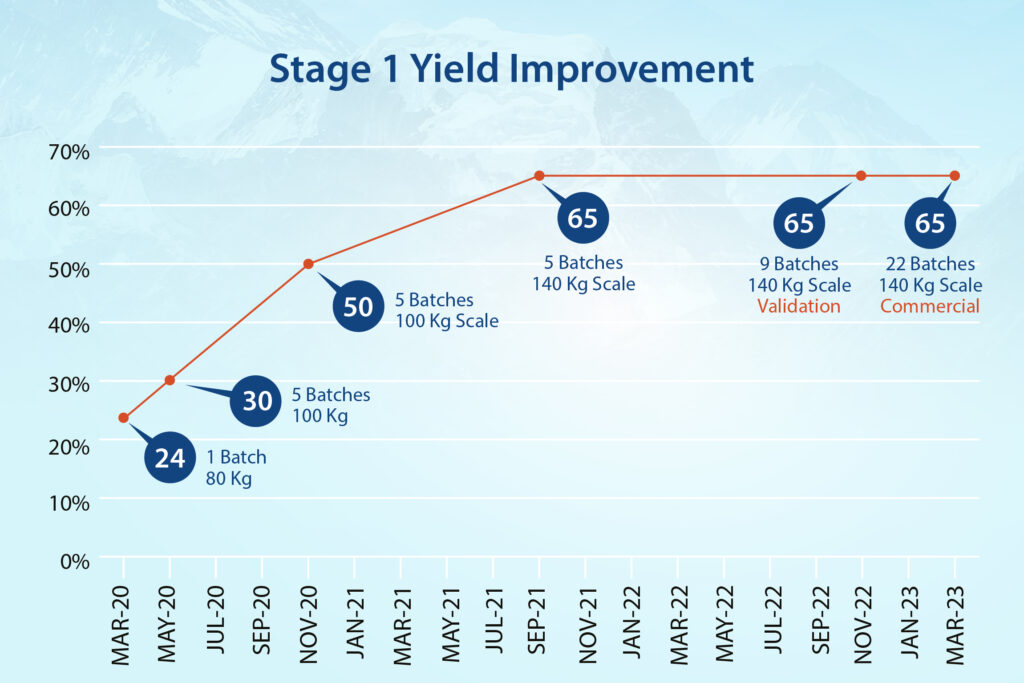
- In Stage-1, the team streamlined hazardous cyanation conditions and achieved a 170% improvement in yield.
- In Stage-2, a hazardous base was replaced with an eco-friendly option while reducing reaction times from two days to a few hours and improving yield consistently.
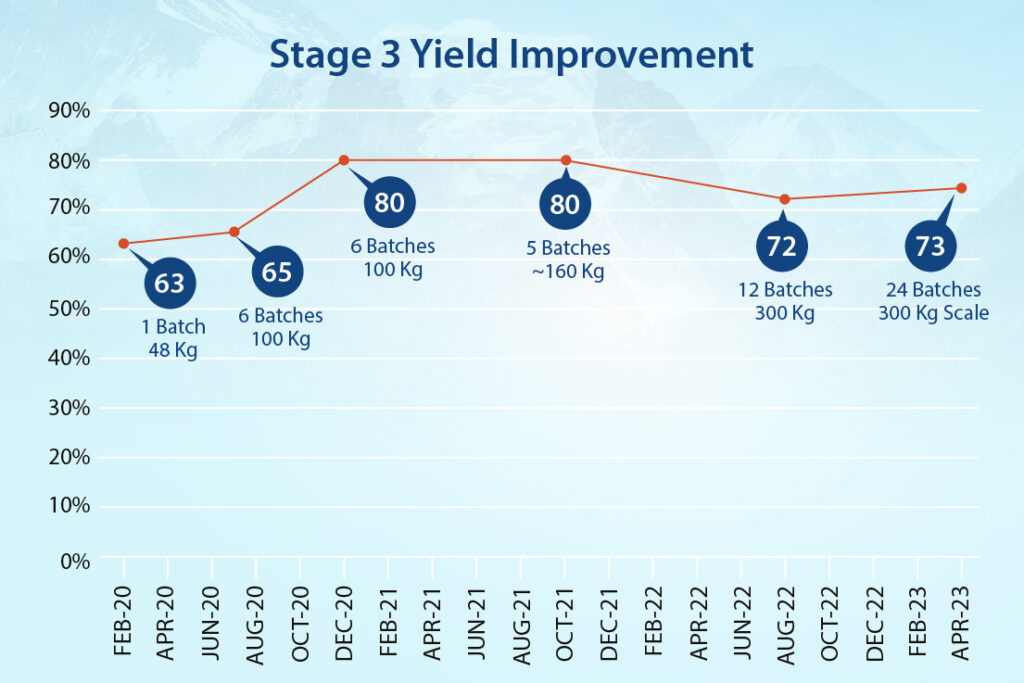
- Stage-3 produced additional efficiencies, with work-up timelines minimized and multiple filtration steps removed. We improved yield at this stage by 16%.
- In the final stage (Stage-4), a purification method was designed to achieve higher quality material. And also include it as a possible reprocessing option.
Other Project Highlights Included:
- Impurity profiling (e.g., genotoxic impurities, nitrosamines)
- Polymorph screening and particle size distribution
- Design of Experiments (DoE)
- Failure Modes and Effects Analysis (FMEA)
- Analytical method development and validation.

Comprehensive Team Approach
Quickly approaching timelines required frequent collaboration from Neuland and Karuna, including process chemistry, analytical development, process engineers, Quality, EHS, production, and export logistics teams. A collaborative approach ensured seamless information-sharing which accelerated and simplified scaling.
Given the global and process-related complications – the project advanced swiftly.
- Following the initial 3-4 kg., production of the API by the previous CMO, by early 2020 Neuland was producing 25-kilogram batches.
- Less than 6 months later, scale-up had advanced to 50 kg. batches.
- NDA registration batches and a multi-batch run of 220 kilograms for Phase 3 clinical trials were then produced.
- This was followed by Design of Experiment (DoE) studies and analytical method validation.
- Four 600 kg. PPQ batches were manufactured to confirm process control and validation.
- In 2022, Neuland began manufacturing multi-metric ton launch quantities for Karuna.
Successful Design, Development and Launch
While API projects often feature one or two particularly strong outcomes, this project hit nearly every high point imaginable – from successful scale-up on abbreviated timelines, improving cost efficiencies, meeting regulatory objectives, and improving the client’s (and our own) supply chain security.
- Overall process yields were improved 3-fold.
- Project costs were reduced by 60%.
- Hazardous reagents and reactions were minimized.
- Processes were optimized, reducing the number and duration of key tasks.
- The process was scaled to multi-hundreds of kilograms.
Contact us to learn how Neuland can make your next NCE API project a success, write to Saradhi@neulandlabs.com










