API particle size is a key consideration in manufacturing quality pharmaceutical products, however, particle size is also notoriously difficult to control during manufacturing. Despite the difficulty, it’s essential to get right: particle size and morphology are the two most significant properties in oral solid dosage manufacture and performance, according to research published in AAPS PharmSciTech.
This post explores why API particle size matters and how API characteristics influence particle size. The next post in our series will highlight the emerging technologies for managing particle size.
Why does API particle size matter?
In the pharmaceutical industry, particle size in APIs is a critical factor. This essential parameter has a direct influence on characteristics such as:
- Absorption behaviour
- Content uniformity
- Dissolution
- Flowability, which in turn impacts the end product’s efficacy, bioavailability, and shelf life
 Those characteristics all affect the end patient, but API particle size also matters on the manufacturing side. API particle size, as well as particle size distribution, can affect the ease of the manufacturing process and the yield per batch.
Those characteristics all affect the end patient, but API particle size also matters on the manufacturing side. API particle size, as well as particle size distribution, can affect the ease of the manufacturing process and the yield per batch.
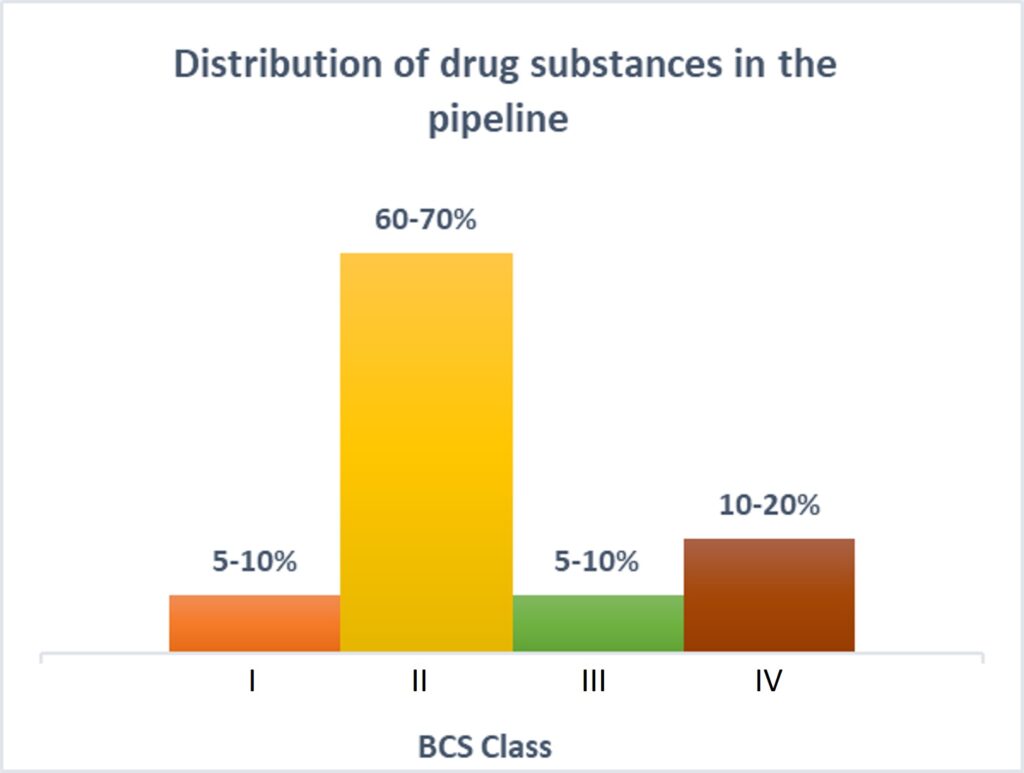
Another primary reason to have precise control over particle size is solubility. More than 80% of the new chemical entities (NCEs) in the current pipeline belong to BCS class II and class IV, making the future of pharmaceutical products look insoluble!
Particle size is an essential characteristic to creating a successful pharmaceutical product, but it’s a notoriously difficult aspect to control during the manufacturing processes. Even a small change in crystallization conditions, such as RPM/agitator/solvent ratios and cooling rates, can lead to significant changes in API particle size, as well as contribute to other property changes.
Another significant factor is changes in an API supplier, which can lead to new particle characteristics. If your company begins working with a new API supplier, be sure to assess the risks leading to changes in particle size. Carefully evaluate whether those changes will affect the drug properties, and if so, to what extent. If the new API size will affect drug properties, see if the method for characterization of particles is still valid.
How to manage API particle size
Managing API particle size is one of the most difficult parts of the manufacturing process. API characteristics exert a profound influence on the strategies employed for managing particle size; therefore, different kinds of particle sizes may require different methods of handling. Here are some examples of characteristics of APIs that can help manufacturers manage the particle size carefully.
· Solubility
The solubility of an API dictates the bioavailability of a compound. APIs with low solubility demand a reduction in their particle size, thereby increasing their surface area and improving drug’s dissolution properties. To manage API particle size, manufacturers can use various reduction techniques, such as micronization, different types of milling, and nanosizing. These methods can be used to decrease particle size and increase solubility.
· Crystallinity
Crystalline structure plays a pivotal role in bioavailability. Amorphous APIs (devoid of crystal lattice) exhibit higher solubility and bioavailability compared to their counterparts. Therefore, preserving a desired amorphous state becomes critical and provides a precise control over particle size.
· Chemical Properties
APIs with inherent chemical reactivity can pose unique challenges in maintaining particle size during manufacturing processes. Different conditions, such as lump formation during drying or a hygroscopic nature, can lead to the formation of an agglomerate, which frequently results in a higher particle size. To manage API particle sizes for chemically reactive compounds, manufacturers must control environments by using dehumidifiers and specialized equipment to ensure integrity of the final product.
What types of API characteristics make particle sizing projects more difficult or complex?
 Managing the size of API particles is typically a complex process, but certain API characteristics are even more challenging to manage than others. Here are four characteristics that can make particle-sizing projects more difficult.
Managing the size of API particles is typically a complex process, but certain API characteristics are even more challenging to manage than others. Here are four characteristics that can make particle-sizing projects more difficult.
1. Particle size distribution (PSD)
Many projects involve products with a single tier of PSD requirements. For example, a product may be d90 < 10 microns, which means that 90% of the particles are 10 microns or less. As with any pharma project, challenges can arise even during single–tier requirement products.
Projects become more challenging with two-tier requirements, in which materials are required to meet two distinct specifications. As the specifications move to three tiers, the difficulties become even more magnified. Controlling the particle sizes—for example, matching between the various tiers of d50/d90—can require the study of a combination of crystallization process and different particle reduction and sizing techniques.
2. Polymorphism
Polymorphism refers to the ability of a compound to exist in multiple crystalline forms, known as polymorphs. Polymorphs have variations in physical properties such solubility rates, melting point, density, flowability, and particle size distribution. One polymorph may have larger particles or dissolve more readily compared to another.
3. Crystallization
Crystallization may also make an API more challenging. Crystallization challenges can involve precise temperature, solvent selection, and other parameters, thereby adding an extra layer of complexity to the process. Since crystallization can affect different pharmacokinetic properties, extensive studies are required to ensure the selected form does not change its particle size or stability over time. All these necessitate precise control over the manufacturing process to achieve consistent particle sizes.
4. Hygroscopicity
APIs which are hygroscopic in nature can absorb water molecules. They are prone to moisture uptake from environmental air conditions leading to changes in the particle size. This can create agglomeration or clumping, causing non-uniform PSD. Moisture can also lead to the degradation or physical instability of the API.
Hygroscopic APIs are difficult to process for size reduction, as the materials tend to absorb the moisture and not have the flowability to pass through the mill. Manufacturers should ensure controlled environments with low humidity levels along with moisture-resistant packaging and desiccants to maintain desired particle size.
Stay tuned next month for part two of our series on API particle size. If you’re currently having challenges managing particle size and have questions, contact us here.










