Technology transfer from pharmaceutical companies to contract development and manufacturing organizations (CDMOs), is critical to successful drug development. When you consider the skills, knowledge, intellectual property, technologies, and manufacturing methodologies being transferred, it’s easy to comprehend both the importance and complexity of such an undertaking.
The expertise needed for an active pharmaceutical ingredient (API) technology transfer requires solid technical proficiency, a specific type of project management skill, commitment to innovation, regulatory knowledge, logical thinking, quality, compliance, adaptability, collaboration, continuous improvement and efficiency that assures R&D professionals, drug development scientists, and key decision-makers in the industry of success. This work is instrumental in shaping the future of drug development and manufacturing through strategic collaborations and advanced technological integrations.
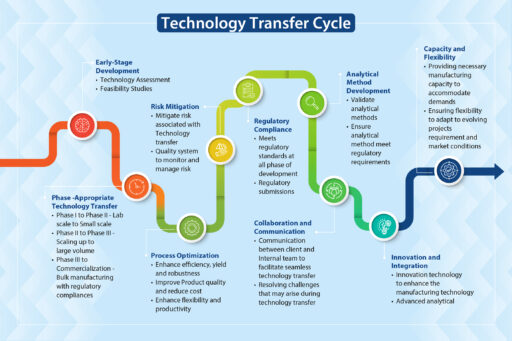
API technology transfer requires a roadmap that clearly directs all the players, in particular the technology transfer team, from one stage to the next. Neuland’s tech transfer process includes the following:
Stage One – Document the Scope and Objectives
The first step is to establish clear communication channels with the client. The synergy of collaboration developed early-on between different departments and teams is the path for a seamless transfer. It fosters a culture of trust and open communication that ensures every stakeholder, from the scientists to supply chain managers, is aligned with, and supports, the project’s goals and timelines.
Second, we agree on the scope and objectives of the project. Once the communication channels are established and trust built, it’s time to finalize the Scope and Objectives of the Technology Transfer. The client and CDMO achieve clarity on what they want and what is possible. There will be a give and take, especially once the baseline assessment is completed. Fortunately, a component of the communication channels includes regular updates and transparent discussions. Because of this process, all parties can adapt quickly to changing needs and expectations. Feedback and open dialogue facilitate the exchange of critical information, assuring every project is tailored to specific client requirements.
We then assess the client’s technology and processes. A detailed assessment identifies:
- strengths and weaknesses
- potential challenges and risks
- the regulatory requirements necessary to attain compliance.
A proactive approach for the assessment means risk mitigation begins quickly – potential challenges are identified early on and we address issues before they become a problem and escalate.
Stage Two – Develop a Detailed API Technology Transfer Plan
The CDMO’s cross-functional project management team is responsible for creating a detailed tech transfer plan customized for each project. Our tech transfer plan includes:
- Defining the project objective, scope, and timeline.
- Identification of key stakeholders and establishing communication channels.
- Creating strong, yet flexible, timelines agreed to by the key stakeholders.
- Developing the technology transfer protocol outlining objectives, scope, methodology, and clear roles and responsibilities, including decision making authority.
- Identification of the resources needed to complete the transfer.
- Process optimization and process validation studies to confirm robustness and reproducibility
- Gap analysis and developing strategies to address gaps and ensure compliance with regulatory requirements.
- Regulatory expectations that must be met to obtain compliance.
- Describing expected quality standards, moving beyond compliance to obtaining real-time data for decision-making.
- Commercialization and post transfer support by ensuring continuity of supply and compliance with regulatory requirements.
Stage Three – Transfer Analytical Methods and Validate Methods
Stage One and Two focus on building the relationship with the client and creating plans to ensure a successful transfer. In Stage Three, we perform analytical testing by developing a comprehensive method transfer protocol outlining the objective, scope, and methodology for transferring and defining acceptance criteria including precision, accuracy, sensitivity, and specificity to prove that the knowledge is successfully transferred.
Method validation includes method demonstration by following regulatory guidelines (ICH Q2(R1)) and documentation which includes method protocols, validation protocols, raw data, results, and conclusions. Analytical method validation during the technology transfer process supports consistent quality and safety of the API at different sites.
Stage Four – Scale Up and Optimization
An effective approach to scale-up is anchored in optimized process development. Scientific expertise is foundational to the development of processes that are not only scalable but also efficient and cost-effective.
Advanced technologies are necessary to meet the growing and evolving needs in global healthcare. They need to be integrated into scale-up strategies. Adopting cutting-edge solutions enhances present manufacturing processes.
Your CDMO should focus on First Time-Right manufacturing. Unfortunately, this is not always emphasized enough. Rework is costly from both a financial standpoint and getting the product to market in a timely fashion. At Neuland, our goal is to avoid rework and streamline the path from development to production by making it right the first time.
Stage Five – Validation Studies
Needless to say, validation of processes and results is a critical component of API technology transfer. We follow robust protocols to determine if each step of the manufacturing process meets the necessary quality standards and is reproducible on a commercial scale. We conduct validation tests, thoroughly analyze the results, and complete a report including any and all corrective actions that must be taken.
Stage Six – Training and Knowledge Transfer
Training and the transfer of knowledge is a thread through all stages. When collaborating with a client on a complex technology transfer the transfer of knowledge starts on Day 1. In Stage Six, the training and knowledge transfer becomes more structured. Standard Operating Procedures are documented and taught. Quality procedures, concepts, and skills such as Continuous Improvement are documented and presented.
Stage Seven – Trial Batch and Verification
After the technology and knowledge is transferred it’s time to test whether the transfer is doing what it is supposed to do by running a trial batch of the product. The batch must be analyzed and verified that it meets the quality specifications and regulatory requirements. The ultimate question needs to be answered – is the API safe to scale up and go to market?
Stage Eight – Regulatory Submission
Meeting global regulatory standards is necessary if your product is ever going to get to market. An unwavering commitment to quality and compliance is at the core of a seamless, successful technology transfer process. Meeting and exceeding regulatory requirements while ensuring processes align with global standards is crucial. At Neuland, a focus on regulatory compliance is integrated into every phase of a technology transfer, from initial planning to final production, ensuring the highest level of integrity and excellence.
When the roadmap is assiduously followed by the cross-functional project management transfer team, they consider, implement, and meticulously document procedures throughout the stages. All procedures are designed to meet and exceed global regulatory standards. At this stage, the documentation is aggregated, formatted, and submitted.
Stage Nine – Continuous Improvement
Continuous improvement (CI) is the crux of maintaining a market lead. Continuously using feedback and data-driven insights to refine processes will maintain excellence. CI starts early, continues through the transfer process, and becomes on-going after the transfer process is complete.
Stage Ten – Technology Transfer Closure
Upon successful completion of all the stages and attaining compliance, the technology transfer cycle is closed.
Leveraging Digitalization for Seamless Tech Transfer, Data Integration
Digital transformation is revolutionizing our industry and new technologies are being used to enhance API manufacturing and technology transfer processes. The adoption of cutting-edge digital tools and technologies streamlines the transfer of information, optimizes processes, and improves overall efficiency from API development to production or additional manufacturing sites. Digital platforms allow high precision and control throughout manufacturing processes, ensuring compliance and consistent quality.
In addition to smooth communications among teams, digital technologies facilitate seamless date integration from various sources across R&D, process development and manufacturing ensuring transparency and real-time updates. These platforms have become a veritable gold mine for the industry improving data visualization and manipulation, increasing the amount of data readily used for decision making and standardizing formats. Collaboration between teams becomes easier and increasingly streamlined.
The ability of today’s process modeling and simulation powers reduces the need for extensive experimental trials to get the same information: an improved understanding of manufacturing processes, the ability to move and optimize parameters, and predict outcomes.
This digital approach expedites the tech transfer process and enhances the quality and reliability of outcomes at your CDMO.
Another proactive improvement is the use of Product Analytical Tools (PAT) to provide for real time monitoring visibility in manufacturing processes. These automated tools reduce manual labor and increase accuracy and reproducibility. Trouble shooting in real time, with ease, stops problems before they create waste.
Support for Your API Technology Transfer
At Neuland Labs, we have a long history of successful API technology transfers and possess a deep understanding of all of the elements of a successful technology transfer and its challenges. We have a proven track record of innovative solutions. Ask us about them!
We leverage our experience in technology transfer, digital transformation, risk management, compliance and robust quality systems, to assist your scale up endeavors. Our experience in this field gives us the ability to anticipate and prevent or solve problems of any complexity. Our goal: a scale up that meets your needs and runs smoothly each time.
Contact us to learn more about how we can help you.










