
Regulatory Affairs
Neuland is committed to manufacturing products in total compliance with regulatory requirements and customer expectations. All process and manufacturing operations are in accordance with cGMP requirements, US FDA and ICH guidelines and regulations.
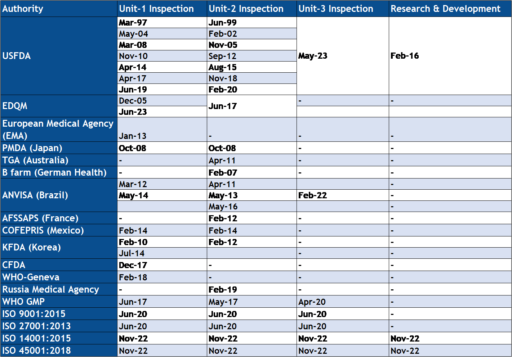
The Company has now filed 66 DMFs with the USFDA, over 499 DMFs in Europe, and many more with the various health authorities in Canada, Japan, Korea and Australia.
The Company supports customers with complete documentation including Drug Master Files for their dosage form applications/ANDA filings.










