 Supply chain management is an essential chapter in the pharmaceutical manufacturing handbook. Managing regulatory starting materials (RSMs) seeks to maximize commercialization and sustainability while meeting and/or exceeding regulatory requirements.
Supply chain management is an essential chapter in the pharmaceutical manufacturing handbook. Managing regulatory starting materials (RSMs) seeks to maximize commercialization and sustainability while meeting and/or exceeding regulatory requirements.
Drugmakers must also ensure the final product conforms to the non-negotiable, pharmaceutical standards of excellence relating to quality, safety, and efficacy.
Admittedly, it’s a fine line between advancing the priorities of regulatory authorities and the interests of stakeholders.
This two-part blog series offers an exploration of the critical stages of sourcing and supplier management of regulatory starting materials.
1. Supply Market Analysis
Supply market analysis sets the stage for identifying and qualifying a supplier of RSMs. It develops an understanding of the essential factors of the market, and the information helps formulate the right 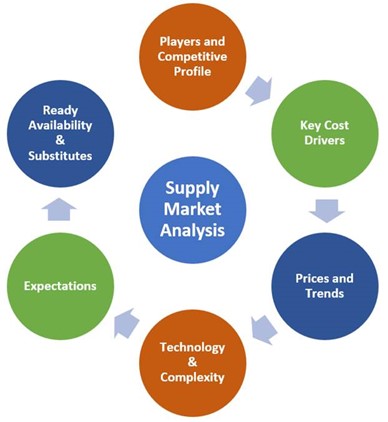 sourcing strategy for future, large-scale procurement initiatives.
sourcing strategy for future, large-scale procurement initiatives.
Because of this, supply market analysis should be considered obligatory before building new collaborations, especially if there’s a high degree of risk and/or value chain competitiveness. Generally, the rewards outweigh the resource investment for this analysis. Supply market analysis involves the following steps:
- Breaking down the market structure. The first step is identifying product category segments. Determining how the market works also includes determining the market size, key drivers, and other market conditions.
- Establishing a market competitive profile. Studying the competitors reveals supplier market share, price variances, and whether there’s a need for substitute materials if the supply-demand trend is off-kilter due to monopoly. This information facilitates strategic decision-making.
- Understanding the supply chain. The supply chain has many moving parts, and each portion has its own risks and values. In our (perhaps) post-pandemic world, there are significantly more uncertainties and complexities than there were before. Understanding the supply chain allows for the development of a tailored procurement strategy.
Basic manufacturer requirements of the soon-to-be-procured material should already be in place to ensure a narrower focus and efficient data gathering. The requisite details include product and material specifications, quantity, and regulatory classifications.
Things to Consider
- How readily available is the required material?
- What are the emerging trends in the market?
- Who are the key players and what are their key weaknesses/strengths?
- What is the competitiveness level among key suppliers?
- What does the pricing landscape look like?
- What is the overall capability and capacity of the market?
- Are there other factors that shape the market, such as geography?
2. Feasibility Studies and Laboratory Validation
Supply market analysis should result in the beginnings of a viable supplier list. This, in turn, is a solid starting point for selecting suppliers and starting materials. Before manufacturing a new product, feasibility studies and lab validation are paramount.
 Feasibility studies and laboratory validation are used to assess whether starting materials are suitable. Feasibility studies shed light on the costs, routes, purity levels, process criticalities and technology deployment for the possible routes.
Feasibility studies and laboratory validation are used to assess whether starting materials are suitable. Feasibility studies shed light on the costs, routes, purity levels, process criticalities and technology deployment for the possible routes.
Suitable samples must be procured, assessed, and used to define the preferred route of synthesis. These steps define the number of critical process stages, the ease or difficulty of impurity purging, and the overall risk from start to finish.
After performing multi-batch feasibility studies and finalizing experimental work and production trials, a comprehensive validation report is compiled and signed off on. The next step is selecting the right RSM for procurement.
Things to Do
- Establish a validation protocol with scope boundaries.
- Procure sufficient samples of various structural fragments with Certificates of Analysis.
- Assign a qualified and trained R&D team to perform lab experiments to ensure validation of material specifications.
- Use lab experiments to support the development and evaluation of the route of synthesis with different, readily available starting materials.
- Leverage information from feasibility studies and validation reports to negotiate prices and keep the value chain competitive.
3. Source and Starting Material Selection
There’s a crucial connectivity between selecting suitable regulatory starting materials, and feasibility studies and laboratory validation. Remember, the validation stage involves the analytical testing of procured samples ahead of commercial production.
- This analytical testing of samples checks if all material specifications are properly defined. It also paves the way for finalizing in-house development of critical steps representing significant moiety of the drug substance.
- From there, all that’s left is finalizing input specifications and narrowing down targeted suppliers.
4. Source Selection
This stage of starting material designation and justification should arrive at a finalized shortlist of at least three suppliers. Generally, source selection uses wide-ranging acceptance criteria that cover the following:
- cGMP
In case of Regulatory Intermediates, aspiring suppliers require cGMP certification from a relevant medical authority. For supply of key starting materials, a GMP-like production environment and the application of standard industry controls to manufacture pharmaceutical intermediates is called for. Starting materials should also adhere to appropriate pharmaceutical industry controls. For large-scale manufacturing, suitable sources should have ISO14001, and ISO 18001 certifications as needed.
- Market position
Quality assessment of sources within the supply market looks at industry and product experience, overall reputation, technical and innovative competencies, current client base, and more. Using data generated during market analysis also helps distinguish top suppliers who can guarantee better end-product quality and compliance.
- Supplier capacity and capability
Analyzing supplier capacity and capability is critical for assurance of supply. Many factors come into play here, including infrastructure, financial stability, complex chemistry know-how, and more. - Procurement cost-efficiency
Once cGMP status, capacity and quality are ascertained, source competitiveness becomes a key factor. Competitiveness balloons in importance as manufacturing processes are scaled. While there may be room for cost improvement negotiations down the line, it never hurts to pay attention at earlier stages.
Selection Checklist
- Quality and regulatory compliance. Consider the supplier’s compliance track record, quality management systems, production infrastructure, and documentation processes.
- Cost procurement dynamic. Review costs and determine how to achieve the desired cost-effectiveness level during the commercial phase. For instance, it’s possible to reduce costs through scrutinizing emerging markets and implementing cost management throughout.
- Quality of service. Confirm lead times and assess communication, responsiveness, and other service quality measures, such as expertise, competency, and innovation.
These are some of the basics of supply management. In Part II, we’ll cover the later stages of sourcing and supply management with regard to regulatory materials.










