Have other areas in drug discovery suffered because of COVID-19?
 It’s probably not too early to say that COVID-19 has changed pharmaceutical drug discovery considerably. And why wouldn’t it have, since it changed almost everything else?
It’s probably not too early to say that COVID-19 has changed pharmaceutical drug discovery considerably. And why wouldn’t it have, since it changed almost everything else?
Roaring onto the scene in the first quarter of 2020, COVID-19 proceeded to shut down entire nations and continents in the second quarter, and now continues to dominate world news, global economies, and our everyday lifestyles (“Should we go shopping? Eat out? Return to campus?”) in the 3rd quarter.
So, yes…of course it has had an impact on drug discovery and development. What remains to be seen is whether it will be an enduring transformation or if we will return to a semblance of ‘pre-COVID normal’ at some point.
In June, Forbes captured the scope of the coronavirus challenge and the reason why we are seeing such an unparalleled effort to defeat a single disease:
“In the last eight months the pandemic has emerged with stunning suddenness to infect more than 6.5 million people world-wide—killing nearly 400,000. Cambridge University estimated that losses over the next five years would total nearly $27 trillion, more than 5% of global GDP. As the world hopes for salvation, how pharma responds will have a profound effect on its future.”
Emphasis: Coronavirus
By March 2020, drug discovery and development efforts had started to coalesce around two broad medicinal objectives: (1) find suitable therapeutic compounds & modalities for treatment, and (2) develop a vaccine.
These were not mere add-on discovery programs. Assets and resources were re-purposed from a variety of sources, and companies, organizations and governments were given free rein to find a treatment or cure.
In an article at Nature.com on how the coronavirus outbreak could make it quicker and easier to trial drugs, Kenneth Kaitin, director of the Tufts Center for the Study of Drug Development in Boston, Massachusetts discussed how the pandemic has touched nearly all aspects of the industry. “This has really turned upside down the whole drug-development process,” he says. “The entire investigative world is focused just on developing treatments for COVID-19.”
Single-Minded Global Focus on COVID-19
The world has adopted a singular focus – which is a rarity in drug discovery circles. Governments and organizations have, in the past, driven focus in specific therapeutic areas – think ‘cancer moonshot’ – but never to the exclusion of other development efforts as we are seeing today.
That’s not to say everything has stopped. While coronavirus research is taking precedence and has had an impact, other research continues, patents are being filed and deals are getting done. It does feel as though non-coronavirus news is sliding under the radar, and it’s to be expected: we’ve rightfully become preoccupied with COVID-related developments.
More notable than the global focus has been the intensity of worldwide, cross-border and cross-company collaboration. Industry watchers point to this extraordinary level of collaboration, in which the world’s best minds are sharing hypotheses, data, results and more – as perhaps being decisive in the fight against SARS-CoV-2. At the very least, such a model will significantly reduce duplicative efforts and compress timelines in the search for treatments or a vaccine.
Sharing SAR-CoV-2 and COVID-19 Resources
Drug discovery efforts in the fight against COVID-19 have benefitted from never-before-seen levels of information sharing. Collaborativedrug.com has a running list of the many shared COVID-19 drug discovery resources available, driven by both the global urgency to fight an emerging disease and a new model of open source scientific discovery:
“Publishers like the British Medical Journal (and in a moment of solidarity other publishers like Wiley and Elsevier) are providing information on the Coronavirus freely on the internet to spur short-term global response efforts and support long-term research.”
The list of organizations dedicated to working together is unprecedented. Just two of the initiatives include:
- The COVID-19 Open Research Dataset (CORD-19): Allen Institute for AI, Chan Zuckerberg Initiative (CZI), Georgetown University’s Center for Security and Emerging Technology (CSET), Microsoft, and the National Library of Medicine (NLM) at the National Institutes of Health.
- The COVID-19 Therapeutics Accelerator—launched by the Gates Foundation, Wellcome, and Mastercard. Companies participating in the collaboration include: BD, bioMérieux, Boehringer Ingelheim, Bristol-Myers Squibb, Eisai, Eli Lilly, Gilead, GSK, Johnson & Johnson, Merck (known as MSD outside the U.S. and Canada), Merck KGaA, Novartis, Pfizer, and Sanofi. Companies have agreed to share their proprietary libraries of molecular compounds that already have some degree of safety and activity data.
Impacts Beyond Drug Discovery
The discovery of novel therapeutic candidates is just one facet of the broader pharma industry, albeit a vital – and highly-visible – one these days. The impacts of coronavirus, however, have been felt across the breadth of the industry – from supply chain disruptions to later-stage clinical trials.
 On the clinical trial front, COVID-related disruptions have become the rule rather than the exception.
On the clinical trial front, COVID-related disruptions have become the rule rather than the exception.
“Since early March, hundreds of organisations that are acting as the sponsor, collaborator, or contract research organisation (CRO) have publicly announced disruptions to planned and ongoing clinical trials in their press releases, Securities and Exchange Commission (SEC) filings, and clinical trial registries, as well as on social media. Companies have delayed the initiation of planned trials or withdrawn these completely, as well as suspended enrollment in ongoing trials or terminated these trials.”
Most disruptions of clinical trials were due to suspended enrollment, followed by slow enrollment and delayed initiation. The availability of clinical trial sites (including hospitals) and investigators (many shifted efforts to COVID-related drug discovery or treatment) was severely curtailed, hampering trials. All told, about 1,000 organizations (and perhaps more at the time of this post’s publication) had publicly reported disruptions.
What are the implications? Without data from pivotal clinical trials, new drug filings will be delayed, meaning some important new medicines will take longer to reach the market.
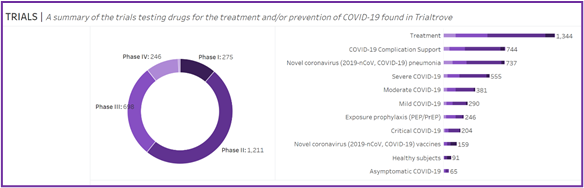
Clinical trials, however, didn’t collectively just end. Thousands of COVID-19-related clinical trials are now ongoing, focusing on treatment of mild, moderate and severe cases, exposure prophylaxis, complication support, asymptomatic cases, critical care and vaccines. And – as mentioned above – other non-COVID-19 related work continues (including clinical trials), despite societal attention being focused elsewhere.
Setting Expectations
The pharmaceutical industry – for all its very public regulatory surveillance and consumer-facing marketing – has always been somewhat opaque. Companies historically have never updated the public on day-to-day progress in the lab.
Why?
It’s about setting expectations. Remember, for every 5-10,000 chemical compounds, only 2-5% will show any promise. Less than half of one percent will qualify for testing on humans, and – of that 0.25-0.5% which begin clinical trials – less than one in ten will be successful. From start to finish, that’s an overall success rate of something on the order of 0.05%. Announcing you’ve identified a chemical compound which could successfully treat a disease would not be rational expectation setting.
The COVID-19 era has seen a sea change in how we set expectations. Johnson & Johnson is even planning to air a reality television series showcasing their COVID-19 efforts. As STATNews noted in a May article: “The narrative emerging from the Covid-19 pandemic is that the market is responding to rescue us from global catastrophe, a public relations coup for an industry that has long known about the potential for another pandemic but hasn’t meaningfully invested in research until now.”
However, as Forbes noted in their June article, this poses a serious challenge for the industry. When NIAID Director Anthony Fauci expressed hopefulness that a vaccine could be produced by the end of 2020, many in the pharma industry thought it over-optimistic. The ‘world record’ for vaccine development – at six years – is currently held by Ebola. Equally concerning to those who want to manage public expectations: to date there has never been a successful vaccine for any coronavirus – whether SARS, MERS or the common cold.
At the same time, however, there has never been such a massive global collaborative effort to find one. And there is some reason to be optimistic. After all, the pharma industry is a 150-year-old chronicle of successful ‘firsts’…stretching from morphine to metabolomics. Innovation and discovery are the actual purpose of our industry.