
As an API manufacturer, when it comes to product safety in the pharmaceutical space the stakes couldn’t be higher. We’ve written a few posts recently on modifications to regulatory inspection regimes (here and here) that are likely to impact – in some fashion – global drug manufacturers.
In their Life Sciences Regulatory Outlook, Deloitte writes: “Life sciences companies exist to help patients and save lives. Regulatory compliance provides guardrails to ensure all companies play by the same rules.”
Well stated, Deloitte. We’re in the business of making drugs that improve people’s lives, and regulators are there to ensure we’re doing it safely in accordance with current best practices.
The last few years have seen major changes to the ways in which drug companies and their regulators go about ensuring the safety and efficacy of drugs. We’re in an era of significant change: scientific knowledge – whether human biology or complex synthetic chemistry – continues to advance.
Most companies are undertaking (and some have completed) the transition to digital data collection and retrieval. Processes are being streamlined and automated wherever possible.
Modernization is affecting everything from analytical instrumentation and process monitoring to risk management practices. Globalized supply chains continue to expand, even in light of recent trade uncertainties.
All of these factors – globalism, science & technology advances, the shift towards digitalization – play a role in the growing ‘complexity of compliance’…as well as the importance of compliance.

A commitment to regulatory excellence matters not because a failure can be expensive (it can, both both financially and in terms of reputation), but because – as Deloitte pointed out – we are in the business of improving people’s lives.
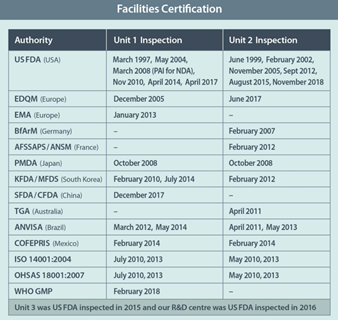
At Neuland, we’re proud of our quality accomplishments and quality system capabilities. We have a strong regulatory track record, with all three of our manufacturing units inspected and approved by regulatory agencies from around the world. (Our Unit 1 and Unit 2 manufacturing facilities have respectively undergone six U.S. FDA inspections.)

How Neuland Labs Creates & Maintains a Pharma Quality Culture
To encourage regulatory excellence, we’ve fostered a culture in which quality & compliance are top priorities across all of our sites, 24X7. The key to ensuring a culture rooted in regulatory excellence lies in processes & procedures – driven by a motivated, quality-focused team. Among the practices we’ve adopted:
- Quality Metrics by Management
We hold weekly Quality Reviews at the plant level. These are led by the Quality Head with Plant, R&D, and RA teams. Monthly Quality reviews occur with top Management (including the CMD and CEO).
- Continuing cGMP Education
Continuous cGMP trainings are conducted with relevant employee teams to maintain up-to-date knowledge in accordance with current guidelines and regulations.
- Constant Audit Readiness
At Neuland, we experience approximately 100 customer audits each year at our facilities. While some companies approach audits or inspections as ‘special events’ which demand the team’s attention immediately prior to the audit, we’ve opted for an ‘all-the-time-audit-readiness’ approach. To this end, we perform continuous QA/CQA audits (both scheduled & walk through), and expect our units to be prepared for audits at any time, without forewarning.
- Commitment to First Time Right
Neuland’s robust technology transfer system was established to achieve First Time Right processes in our The first time right principle focuses on ensuring that “activities are carried out in the right manner the first time and every time.”
 Many of the Quality behaviours we’ve established are rooted in process and technology. For example, we employ:
Many of the Quality behaviours we’ve established are rooted in process and technology. For example, we employ:
- Quality system automation: Electronic Quality Management systems have been implemented for Deviation, Change Control, CAPA, and Complaints across Neuland Units.
- Routinely updated SOPs: Harmonised Procedures/SOPs & Systems are in place across all of Neuland’s Units. We ensure that SOPs are updated in line with current guidelines.
- The latest most compliant instrumentation: Neuland’s Quality Control laboratories are equipped with the latest equipment and systems – including HPLCs, GCs, IR, UVs, SORs, KF titrators and more – to ensure compliance with 21 CFR Part 11 requirements.
- Robust batch release systems: Our batch release systems ensure a thorough review of batch processing and analytical documentation by the site’s Quality Assurance department, in close cooperation internally between departments.
Would you like to read more about Neuland’s commitment to Quality culture? Learn about our Regulatory Affairs department, our Quality Control & Assurance team, or Contact Neuland Labs.










