In our PE Lab, a team of 27 highly specialized engineers integrates the key attributes of QbD process understanding, process control, and continuous improvement with advanced equipment, Design of Experiments Software, and Design Space methodology.
The objective? To optimize process design, develop inherently safer process using the principle of QbD by DOE based on process safety studies for cost competitive and safer commercial process to improve productivity.
At Neuland, our clients have ready access to our fully operational, dedicated Process Engineering Lab (PE Lab). The lab features state-of-the-art instruments, systems and innovative devices to support operations and safety studies using a QbD approach.
Equipment includes a stirred, controlled HEL reaction calorimeter that measures the rate of heat release during reactions along with gas release if any during the reaction. Automated parallel HEL reactors enable multiple experiments to be carried out at temperatures ranging from -60 to 225oC. The lab’s new Thermal Screening Unit (TSU) indicates the thermal stability of chemicals and safe processing temperatures. Ideal for risk analysis, the TSU uses only 0.5-5 g of a sample.
Solving Customer Process Safety & Particle Engineering Issues
In the year since the lab opened, our team has solved numerous process safety and particle engineering challenges for our customers. Highlighted below are just a handful of the exciting projects we’ve taken on.
Anti-Convulsant O6 Process Safety Project
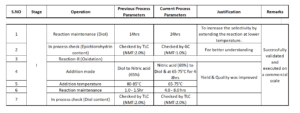
To ensure process safety for the anti-convulsant O6, we optimized the process parameters and demonstrated the process on a commercial scale 350 Kg batch, using PE lab infrastructure data.
We followed this success by filing a patent. The table below details the steps (click to enlarge):
Anti-Tuberculosis Drug Process Safety Project
Another process safety case involved an anti-tuberculosis pipeline drug, a Custom Manufacturing Solution project at Neuland. The customer explained how they were dumping all the reagents and then heating to reaction temperature, as this was scale-dependent.
After thorough evaluation using the TSU, we determined the reaction initiation temperature. The reagent causing exotherm was added at 2°C above the reaction initiation temperature. The batch was produced at plant scale without any problems, then converted from batch mode to semi-batch mode. Automation controlled additions based on both the process temperature and gas release rate.
Predicting the Stability of a Micronized API Project
To meet the physicochemical properties required for an API and optimize particle size distribution (PSD) and bulk density requirements of a final drug formulation, engineers and scientists in the PE Lab leveraged particle engineering techniques and particle size reduction and drying technologies.
They also conducted experiments designed to assess the stability of a micronized API at lab scale. The goal was to be able to predict and – if needed – implement corrective actions to avoid stability-related failure when using a multi-mill, micronizer or fluidized bed dryer to produce commercial batches.
Particle Engineering & Anti-Thrombotic Drugs Project
Our team has also conducted particle engineering experiments using Micronization to meet customer PSD requirements for products such as Indacaterol maleate (less than 5 microns PSD achieved) and Ticagrelor (less than 10 microns PSD achieved). Ticagrelor is used to prevent thrombotic events such as heart attack in people with acute coronary syndrome or myocardial infarction.
Particle Size and Levetiracetam Project
Data generated on experiments with the compound Levetiracetam were used to optimize process conditions to meet the PSD requirement. Having achieved PSD, the different products were dispatched on a Kg scale. Stability testing of the micronized material under real-time/accelerated conditions assessed the impact of the micronization protocol on impurity profiling. Data generated on experiments with the compound Levetiracetam were used to optimize process conditions to meet the PSD requirements.
Concept of Membrane Technology
Reverse osmosis technique is used for concentrating the reaction mass. Dia filtration for removal of salts for cost competitive, scalable process. The concept was used in resolving for API based Amino Acid synthesis and for addressing yield & quality concerns for one of the anesthesia products.
Flow Chemistry & CSTR in series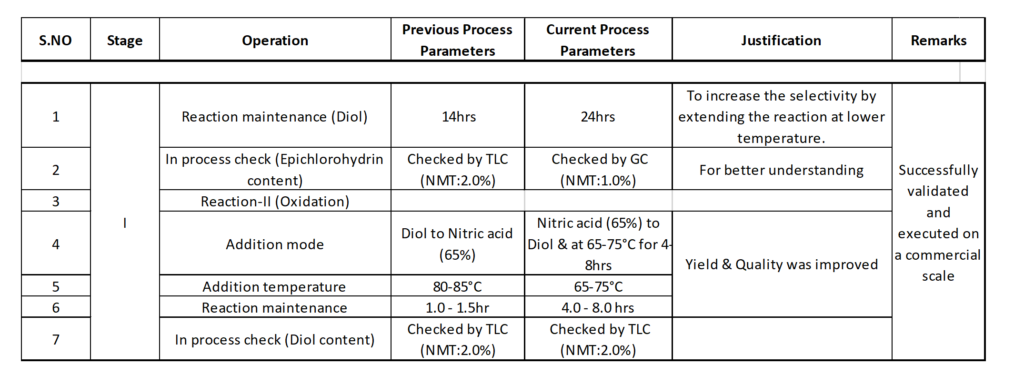 Developed in-house capabilities in flow chemistry and generated proof of concept for handling of hazardous reagents (Strecker reaction NaCN, LDA reaction) at intensified conditions with reaction progressing with less than a minute. Improved Oxidation Reaction Yield and Quality. Executed at plant with desired Yield & Quality., obtained Yield ~65-70% compared to ~30% earlier campaign outsourced. Oxidation reaction done in CSTR’s at lab and obtained acceptable yield and quality. The technology of CSTR’s in series is filed for patentability.
Developed in-house capabilities in flow chemistry and generated proof of concept for handling of hazardous reagents (Strecker reaction NaCN, LDA reaction) at intensified conditions with reaction progressing with less than a minute. Improved Oxidation Reaction Yield and Quality. Executed at plant with desired Yield & Quality., obtained Yield ~65-70% compared to ~30% earlier campaign outsourced. Oxidation reaction done in CSTR’s at lab and obtained acceptable yield and quality. The technology of CSTR’s in series is filed for patentability.
The Problem-Solving PE Lab at Neuland
Data and insights gathered in the PE Lab help our engineers and scientists develop robust processes at lab-scale for new products, ensure inherently safer processes, and understand the complexities of scale-up to enable right-at-first-time technology transfer, minimizing failures at plant scale. Knowledge and data are essential. Well-designed experiments performed in batch mode can test for hazardous reactions and suboptimal unit operations, informing how we define process parameters and controls to implement at scale.
Questions about process engineering? Contact us to see how we can help.