 In a PharmTech webcast, the Neuland team linked up with Dr. San Kiang – Research Professor from the Department of Chemical Engineering at Rutgers University. The objective was a discussion on the importance of collaboration between chemical engineers & pharmaceutical chemists in today’s drug manufacturing environment. This collaboration is important, and is a key element of QbD.
In a PharmTech webcast, the Neuland team linked up with Dr. San Kiang – Research Professor from the Department of Chemical Engineering at Rutgers University. The objective was a discussion on the importance of collaboration between chemical engineers & pharmaceutical chemists in today’s drug manufacturing environment. This collaboration is important, and is a key element of QbD.
I also recently participated in a Q&A specifically on the collaboration between chemists and chemical engineers during drug development. It is a big issue given the colossal changes happening in the drug industry, perhaps most visibly on the quality & regulatory fronts.
Drug Safety, Efficacy and Feasibility
This collaboration can mean the difference between a viable drug and one that had great potential, but was not practical from a manufacturing standpoint. It is customary to evaluate drugs on two pillars common to regulatory environments – efficacy and safety. In other words, does the drug perform what it needs to perform, and does it do it safely?
In the real world of drug discovery, development and commercialization, however, there is a third equally important pillar: feasibility.
 A product can be determined to be safe and efficacious – but if it isn’t feasible to produce (from either an economic or a technical at-scale production standpoint), then it isn’t a candidate for success.
A product can be determined to be safe and efficacious – but if it isn’t feasible to produce (from either an economic or a technical at-scale production standpoint), then it isn’t a candidate for success.
This is especially true since, often before a scalable chemistry process has been fully developed, chromatography (or, more specifically, process chromatography) is used for making materials in early-stage development.
Collaborating Across Scales
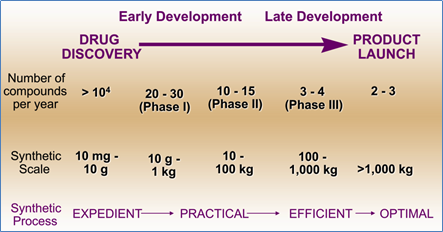
When chemists and engineers work hand-in-hand during process development in R&D, processes tend to progress through scale-up easier. There are considerable differences between producing 10 mg batches and manufacturing 500 kg batches, to be sure – and numerous engineering-related factors need to be taken into account. This chart describes the increasing scales in terms of the synthetic process employed – from expedient, to practical, to efficient and – ultimately – to optimal.
This is the role played by the collaboration of engineers and chemists (and the beauty of QbD, in general): ensuring the smooth transition from the expedient to the optimal while developing a safer process with optimized yield and quality.
Because chemists and chemical engineers approach each challenge from different perspectives, there are different areas of expertise needed.
Chemical Engineers:
- generate data on the material balance.
- evaluate energy balances to understand utility requirements for plant scale.
- select equipment for commercial scale for retrofitting or new, per process requirement.
- perform risk assessments (Quality, Safety) of unit operations, powder safety characterization studies, HAZOP & & HIRA.
- evaluate particle engineering (particle size, bulk density, surface area & Polymorph).
- forecast potential for new technology implementation considering the volume of products, safety threats, troubleshooting activities related to the commercial products, and more.
Chemists:
- leverage expertise in various types of synthetic reactions, based on both literature searches and hands-on experience.
- help select the route of synthesis.
- evaluate the feasibility of the selected route, optimize and validate the process to meet the predefined quality and yield.
- identify and characterize any impurities which have an impact on quality.
- perform generation and qualification of reference/working standards.
- maintain continuous interaction with IP for process infringement with any new process patents,
- perform process and method validation.
Some of the bullets in these lists involve collaboration between the two fields. Chemical Engineers, for example, are involved in process development quite early and play a role in route selection/ finalization. Across the development phase of a project, both chemical engineers & chemists will work together to understand CPPs & CQAs of the process.
More interactions tend to occur once process feasibility has been confirmed and the generated compounds reach a passing level of quality. Once feasibility has been shown, the engineers will evaluate the process from a safety, health and environment standpoint. They then generate process safety data to create inherently safer processes.
When it comes to scale-up, Engineers and Chemists must work closely together to plan the scale up campaign, demonstrate & confirm feasibility and hand over to manufacturing.










